酶催化不对称合成2,3-二氢苯并呋喃酯的动力学拆分及其抗炎活性评价
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用酶作为生物催化剂,通过分子内醛反应生成的2,3-二氢-3-苯并呋喃醇的动态动力学分解,开发了一种高对映选择性手性2,3-二氢苯并呋喃(2,3- dhb)酯的替代策略。该协议提供了方便的访问一系列2,3- dhb酯衍生物,前药,并允许官能团转化。生物学评价也表明,一些产品表现出有效的抗炎活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
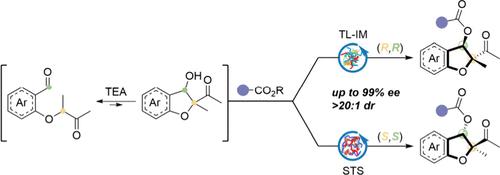
Enzyme-Catalyzed Dynamic Kinetic Resolution for the Asymmetric Synthesis of 2,3-Dihydrobenzofuran Esters and Evaluation of Their Anti-inflammatory Activity
Utilizing enzymes as biocatalysts, an alternative strategy has been developed for the highly enantioselective synthesis of chiral 2,3-dihydrobenzofuran (2,3-DHB) esters via the dynamic kinetic resolution of 2,3-dihydro-3-benzofuranols, which are generated from an intramolecular Aldol reaction. This protocol provides easy access to a series of 2,3-DHB ester derivatives, prodrugs, and allows for functional group transformations. Biological evaluation also indicates that some of the products exhibit potent anti-inflammatory activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


