利用FAD光感受器增强蓝光中质子耦合电子转移
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
使用 FAD 的蓝光(BLUF)光感受器利用非共价结合的 FAD 来吸收光并触发受体激活过程中的初始超快事件。对 BLUF 感光器 PixD 的研究表明,在光周期中会形成黄素自由基(FAD-- 和 FADH--),这支持了 BLUF 的一般运行机制,其中涉及从保守的 Tyr 到氧化的 FAD 的 PCET。然而,在关系密切的 BLUF 蛋白 AppABLUF 和 BlsA 中没有观察到自由基中间体,而且用增加苯酚 OH 酸性的氟-Tyr 类似物取代保守 Tyr 对 AppABLUF 的光激活影响很小,而 PixD 则不同,其光循环在 FAD-- 时停止。BLUF 蛋白中的氢键网络包含几个严格保守的残基,但与黄素 C2═O 相互作用的氨基酸的特性却有所不同。在 PixD 中,C2═O 有两个氢键,而在 AppABLUF 中只有一个。我们利用 TRIR 显示,在 AppABLUF 中,C2═O 上引入第二个氢键会导致在光周期中形成黄素自由基(FAD-- 和 FADH--)。随后用 2,3,5-三氟酪氨酸取代双突变体中的保守酪氨酸(Y21)可防止自由基的形成和光态的产生,这表明 AppABLUF 的光周期现在与 PixD 相似。触发 PCET 的能力从根本上揭示了电子传递在 BLUF 光激活机制中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
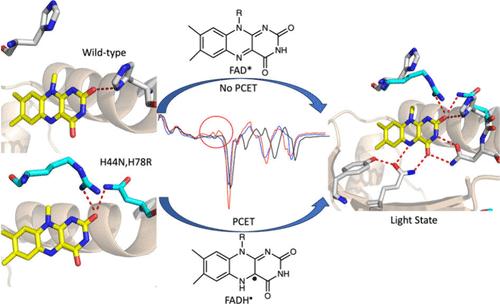
Enhancing Proton-Coupled Electron Transfer in Blue Light Using FAD Photoreceptor AppABLUF
The Blue Light Using FAD (BLUF) photoreceptor utilizes a noncovalently bound FAD to absorb light and trigger the initial ultrafast events in receptor activation. FAD undergoes 1 and 2 electron reduction as an enzyme redox cofactor, and studies on the BLUF photoreceptor PixD revealed the formation of flavin radicals (FAD•– and FADH•) during the photocycle, supporting a general mechanism for BLUF operation that involves PCET from a conserved Tyr to the oxidized FAD. However, no radical intermediates are observed in the closely related BLUF proteins AppABLUF and BlsA, and replacing the conserved Tyr with fluoro-Tyr analogs that increase the acidity of the phenol OH has a minor effect on AppABLUF photoactivation in contrast to PixD where the photocycle is halted at FAD•–. The hydrogen bonding network in BLUF proteins contains several strictly conserved residues but differs in the identity of amino acids that interact with the flavin C2═O. In PixD there are two hydrogen bonds to the C2═O, whereas there is only one in AppABLUF. Using TRIR we show that the introduction of a second hydrogen bond to the C2═O in AppABLUF results in the formation of flavin radicals (FAD•– and FADH•) during the photocycle. Subsequent replacement of the conserved Tyr (Y21) in the double mutant with 2,3,5-trifluoroTyr prevents radical formation and generation of the light state, indicating that the AppABLUF photocycle is now similar to that of PixD. The ability to trigger PCET provides fundamental insight into the role of electron transfer in the mechanism of BLUF photoactivation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


