聚合物骨架在可充电锂电池中硫化聚丙烯腈阴极中的作用
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
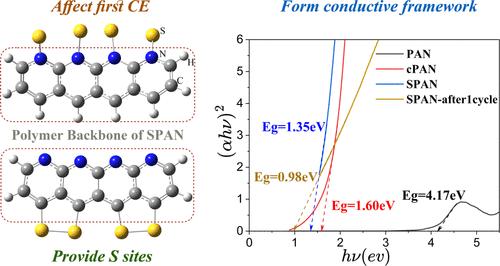
硫化聚丙烯腈(SPAN)由于其非多硫化物溶解性和优异的循环稳定性,已成为下一代锂硫电池极具前景的正极材料。然而,对于构成SPAN聚合物骨架的热解聚丙烯腈的具体作用和影响,人们还没有充分了解。本研究从电化学、光谱学、电镜、理论计算等多个方面对一系列不同硫含量的SPAN材料进行了综合研究。结果表明,聚合物主链至少具有四个关键功能。首先,在SPAN合成过程中,聚合物主链通过化学键为硫的结合提供了反应位点。其次,通过硫的脱氢反应建立广泛的π共轭网络,作为SPAN的导电骨架;化学键合的硫原子附着在聚合物骨架上,缩小了最高已占据分子轨道与最低未占据分子轨道(HOMO-LUMO)之间的能隙。第三,聚合物主链对第一库仑效率起着至关重要的作用,不可逆插入的Li原子进一步掺杂聚合物主链,减小了HOMO-LUMO的能隙。最后,聚合物骨架内的吡啶氮对硫化锂(Li2S)具有吸附作用,从而稳定了SPAN阴极的循环性能。值得注意的是,在1到3v的工作电压范围内,聚合物主链对碳酸盐电解质中SPAN阴极的可逆容量几乎没有贡献。本研究提高了我们对SPAN的认识,为开发更好的SPAN材料和可充电电池提供了有价值的指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Roles of the Polymer Backbone for Sulfurized Polyacrylonitrile Cathodes in Rechargeable Lithium Batteries
Sulfurized polyacrylonitrile (SPAN) has emerged as a highly promising cathode material for next-generation lithium–sulfur (Li–S) batteries primarily due to its non-polysulfide dissolution and excellent cycle stability. Nevertheless, the specific roles and impacts of the pyrolyzed polyacrylonitrile, which constitutes the polymer backbone of SPAN, remain inadequately understood. In this study, comprehensive investigations from multiple aspects, including electrochemistry, spectroscopy, electron microscopy, and theoretical calculations, were conducted on a series of SPAN materials with various sulfur contents. The results reveal that the polymer backbone serves at least four critical functions. First, during the synthesis of SPAN, the polymer backbone provides reactive sites for the incorporation of sulfur through chemical bonding. Second, it establishes an extensive π-conjugated network via a dehydrogenation reaction by sulfur and serves as a conductive framework for SPAN. The chemically bonded sulfur atoms dope the polymer backbone, which narrows the highest occupied molecular orbitals–lowest unoccupied molecular orbitals (HOMO–LUMO) energy gap. Third, the polymer backbone plays an essential role in determining the first Coulombic efficiency, and the irreversibly inserted Li atoms further dope the polymer backbone and reduce the HOMO–LUMO energy gap. Lastly, the pyridine nitrogen within the polymer backbone exhibits an adsorption effect on lithium sulfide (Li2S) species, which stabilizes the cycling performances of the SPAN cathode. It is worth noting that the polymer backbone hardly contributes reversible capacity for the SPAN cathode in carbonate electrolytes within the 1 to 3 V operating voltage range. This study enhances our understanding of SPAN and may provide valuable guidance for the development of better SPAN materials and rechargeable batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


