全氟烷氧基化Ngai试剂及长碳链变体的合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们提出了一种合成恩盖三氟甲氧基试剂和更长碳链变体的直接方法,从而扩大了全氟烷氧基化反应的选择范围。利用具有成本效益的全氟烷基硫酸钠(RfSO2Na)和 N-羟基胺,我们在硝酸铈(IV)铵(CAN)的促进下实现了高效的氧化交叉偶联反应,而无需过渡金属催化剂。事实证明,该方法不仅能有效合成 Ngai 型自由基全氟烷氧基化试剂,还能有效合成 Qing 型亲核型全氟烷氧基化试剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
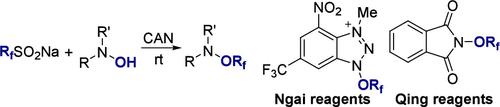
Synthesis of Ngai Reagent and Longer Carbon Chain Variants for Perfluoroalkoxylations
We present a straightforward method for the synthesis of Ngai trifluoromethoxy reagent and longer carbon chain variants, thereby expanding the range of options for perfluoroalkoxylation reactions. Utilizing cost-effective sodium perfluoroalkane sulfinates (RfSO2Na) and N-hydroxylamines, we achieved efficient oxidative cross-coupling reactions facilitated by cerium(IV) ammonium nitrate (CAN) without the need for transition-metal catalysts. The methodology proved effective not only for the synthesis of Ngai-type radical perfluoroalkoxylation reagents but also Qing-type nucleophilic perfluoroalkoxylation reagents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


