戊唑、五磷和环戊二烯阴离子的键合和芳香性:一项综合研究
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究考察了Christe et al.(2002)检测到的五唑酸阴离子([环- n5 -], (a))的π离域、π成键情况和芳构性。为了获得更广阔的视角,我们研究了等π电子种[环- p5 -] (b)和[环-(CH)5 -] (c)。VB分析表明,所研究的三种分子具有显著的共振稳定性,这表明它们具有较高的共振能值。利用电子芳构指数和磁性芳构指数对其芳构性进行了综合分析,结果表明,3种阴离子均表现出较强的π芳构性和较弱的σ芳构性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
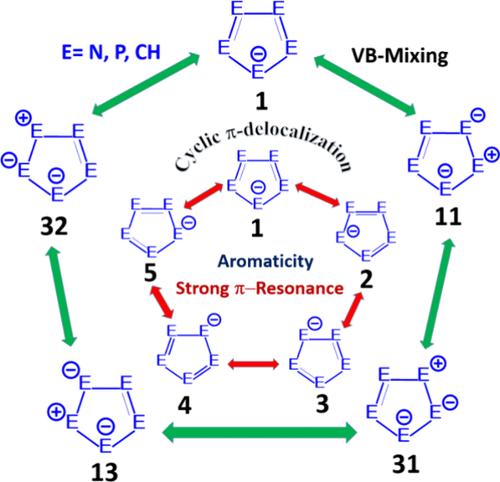
Unraveling the Bonding and Aromaticity of Pentazole, Pentaphosphole, and Cyclopentadiene Anions: A Comprehensive Study
This study investigates π-delocalization, π-bonding situations, and aromaticity of the pentazolate anion ([cyclo-N5–], (a)), which was detected by Christe et al. in 2002. To gain a broader perspective, we investigated the iso-π-electronic species [cyclo-P5–] (b) and [cyclo-(CH)5–] (c). VB analyses reveal that the three studied molecules display significant resonance stabilization, as indicated by their high resonance energy values. A comprehensive analysis of aromaticity was conducted using electronic and magnetic aromaticity indices, revealing that all three anions exhibit strong π-aromaticity and relatively weak σ-aromaticity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


