利用多用途关键对噬菌体展示文库进行大环和双环景观的后期重塑
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
基因编码文库(GEL)正越来越多地被用于发现小分子药物无法解决的 "不可药用 "靶点的配体。噬菌体、酵母、核糖体和 mRNA 显示等基础 GEL 平台已经能够显示由 20 种天然氨基酸(20AA)组成的文库。非天然氨基酸(UAA)和翻译后化学修饰(cPTM)将 GEL 扩展到了 20AA 以外的空间,以产生非天然的线性、环状和双环多肽。标准操作程序是将 UAA 和 cPTM 纳入一个包含 108-1012 个化合物的 "原始 "文库中,然后使用经过多轮筛选的化学升级文库来发现与靶标结合的化合物。然而,这种方法对天然多肽-受体相互作用的了解为零,而在使用 20AA 文库进行筛选时可能已经发现了这种相互作用。关于 "零知识 "的天真文库还是已有知识的文库能为发现分子相互作用提供更有效的途径,目前还没有达成共识。在本手稿中,我们评估了从 "非零知识 "文库中发现大环和双环肽的可行性。我们通过后期化学重塑预选的噬菌体显示的 20AA 与 NS3aH1 蛋白酶的结合情况来解决这一问题。这种重塑使用了一种新型多功能 C2 对称拮抗剂--3,5-双(溴甲基)苯甲醛(称为 KYL),它结合了两个能与硫醇反应的亲电基和一个能与 N 端胺反应的醛基。KYL 将噬菌体显示的肽多样化为双环结构,并划分出两个不同的序列群:(i) 带有 HXDMT 基调的肽,双环化后仍能保持结合力;(ii) 不带 HXDMT 基调的肽,化学修饰后会失去结合力。同样的 HXDMT 家族也可以在从幼稚的 KYL 修饰库开始的传统选择中找到。我们的报告为从已有知识的文库中发现先进的、经过化学修饰的大环和自行车提供了一个案例研究。这些结果表明,在 20AA 空间完成的其他选择活动有可能用于后期重塑,并作为发现高级肽衍生配体的起点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
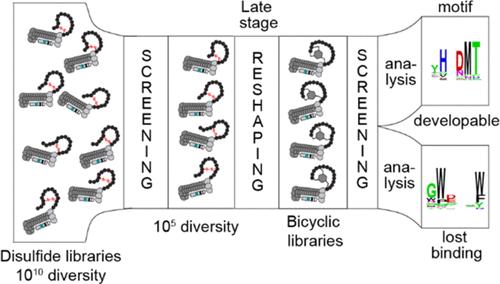
Late-Stage Reshaping of Phage-Displayed Libraries to Macrocyclic and Bicyclic Landscapes using a Multipurpose Linchpin
Genetically encoded libraries (GEL) are increasingly being used for the discovery of ligands for “undruggable” targets that cannot be addressed with small molecules. Foundational GEL platforms like phage-, yeast-, ribosome-, and mRNA-display have enabled the display of libraries composed of 20 natural amino acids (20AA). Unnatural amino acids (UAA) and chemical post-translational modification (cPTM) expanded GEL beyond the 20AA space to yield unnatural linear, cyclic, and bicyclic peptides. The standard operating procedure incorporates UAA and cPTM into a “naive” library with 108–1012 compounds and uses a chemically upgraded library in multiple rounds of selection to discover target-binding hits. However, such an approach uses zero knowledge of natural peptide-receptor interactions that might have been discovered in selections performed with 20AA libraries. There is currently no consensus regarding whether “zero-knowledge” naive libraries or libraries with pre-existing knowledge can offer a more effective path to discovery of molecular interactions. In this manuscript, we evaluated the feasibility of discovery of macrocyclic and bicyclic peptides from “nonzero-knowledge” libraries. We approach this problem by late-stage chemical reshaping of a preselected phage-displayed landscape of 20AA binders to NS3aH1 protease. The reshaping is performed using a novel multifunctional C2-symmetric linchpin, 3,5-bis(bromomethyl)benzaldehyde (termed KYL), that combines two electrophiles that react with thiols and an aldehyde group that reacts with N-terminal amine. KYL diversified phage-displayed peptides into bicyclic architectures and delineated 2 distinct sequence populations: (i) peptides with the HXDMT motif that retained binding upon bicyclization and (ii) peptides without the HXDMT motif that lost binding once chemically modified. The same HXDMT family can be found in traditional selections starting from the naive KYL-modified library. Our report provides a case study for discovering advanced, chemically upgraded macrocycles and bicycles from libraries with pre-existing knowledge. The results imply that other selection campaigns completed in 20AA space, potentially, can serve for late-stage reshaping and as a starting point for the discovery of advanced peptide-derived ligands.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


