氧二聚化驱动的阳离子迁移诱导无序岩盐阴极的电压滞后
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
尽管氧氧化还原有可能提高富锂阴极的能量极限,但其实用性受到伴随的结构变化和电压滞后的限制。虽然电压迟滞通常与过渡金属(TM)迁移和氧二聚化有关,但两者的具体作用尚不清楚。我们提供了一个机制的见解,如何每一个这些变化引起迟滞的代表性富锂无序岩盐阴极,Li1.2Mn0.4Ti0.4O2。我们发现,在电化学过程中,氧二聚体的形成和裂解可以异常迅速地发生,这表明二聚过程不是电压滞后的直接原因,这与流行的观点相反。相反,氧二聚体通过诱导TM迁移间接加剧了滞后,从而导致结构内富二聚体和富TM区域的演化。我们证明了TM迁移比二聚化相对慢,因此在带电电极弛豫期间通过耗散内能和在每个循环中诱导阳离子重排来促进滞后。本文章由计算机程序翻译,如有差异,请以英文原文为准。
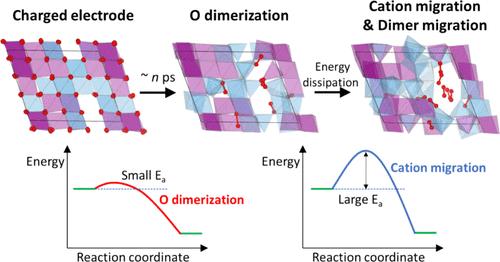
Oxygen Dimerization-Driven Cation Migration Induces Voltage Hysteresis in Disordered Rocksalt Cathodes
Despite the potential to increase the energy limit of Li-rich cathodes by using oxygen redox, its practicality has been limited by the accompanying structural changes and voltage hysteresis. While voltage hysteresis is commonly associated with transition metal (TM) migration and oxygen dimerization, the specific contribution of each is unclear. We provide a mechanistic insight into how each of these changes induces hysteresis in a representative Li-rich disordered rocksalt cathode, Li1.2Mn0.4Ti0.4O2. We reveal that the formation and cleavage of oxygen dimers can occur exceptionally rapidly during the electrochemical process, suggesting that the dimerization process is not directly the cause of voltage hysteresis, contrary to prevailing arguments. Instead, oxygen dimers are found to indirectly exacerbate hysteresis by instigating TM migration, which leads to the evolution of dimer-rich and TM-rich regions within the structure. We demonstrate that TM migration is relatively slower than dimerization and as such contributes to hysteresis by dissipating internal energy during the relaxation of charged electrodes and by inducing cation rearrangement with each cycle.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


