氟促进环丙基碳烯热扩环成宝石二氟化环丁烯
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
宝石二氟化环丁烯的合成对氟原子引入与四元环保留的相容性提出了一定的挑战。在此,我们开发了一个无过渡金属的策略,从宝石二氟化环丙基n-甲苯腙通过扩环反应合成宝石二氟化环丁烯。宝石-二氟取代改变了环丙烷的性质,促进了环丙基碳烯的热重排成环丁烯。该反应可以在没有惰性气体保护的情况下进行,从而为合成宝石二氟化环丁烯提供了一条有效的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
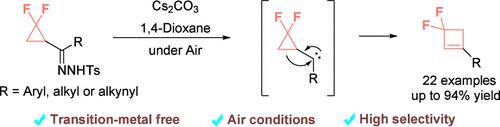
Fluoro-Promoted Thermal Ring Expansion of Cyclopropyl Carbenes to gem-Difluorinated Cyclobutenes
Synthesis of gem-difluorinated cyclobutenes presents certain challenges for considering the compatibility of the fluorine atom introduction with four-membered ring retention. Herein, we develop a transition-metal-free synthetic strategy toward gem-difluorinated cyclobutenes from gem-difluorinated cyclopropyl N-tosylhydrazons via ring expansion reaction. The gem-difluoro substitution alters the properties of the cyclopropane, facilitating the thermal rearrangement of cyclopropyl carbenes into cyclobutenes. This reaction can be easily handled free from inert gas protection, thus offering an efficient route to synthesize gem-difluorinated cyclobutenes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


