水合电催化:提高海水电解中氧进化反应的选择性
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
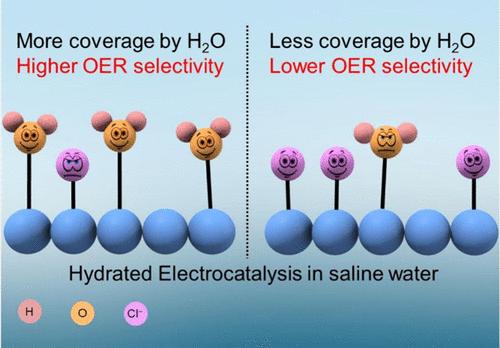
可再生电力生产的增加提供了从使用化石氢向绿色氢过渡的机会。虽然通过水电解大规模生产绿色氢需要大量的淡水资源,但海水的丰富可用性为直接将其用作水电解槽中的电解质提供了一个有希望的机会。然而,海水电解的一个主要挑战是与阳极的氯析出相比,氧析出的选择性较低。为了解决这个问题,我们提出了一种通过水合电催化提高析氧选择性的策略,其中水本身就是催化剂的一部分。与活性催化位点配位的水分子在提高析氧选择性方面起着重要作用。用旋转环盘电极伏安法在酸化模拟海水电解质中对普鲁士蓝模拟电催化剂进行了验证。采用微动力学模型分析了反应物(H2O和Cl -)的覆盖率与选择性之间的关系。值得注意的是,水合电催化剂上的结晶水覆盖率是析氧反应选择性的部分描述符。为了深入了解结晶水和Cl -的覆盖范围,进行了热重分析、Rietveld精馏和微动力学Tafel分析。简而言之,我们探讨的问题是:如果反应物分子(H2O)是催化剂的组成部分,它是否能够促进相应的电化学氧化反应(O2析出)而不是其竞争对手(Cl2析出)?本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydrated Electrocatalysis: To Boost the Selectivity for the Oxygen Evolution Reaction in Seawater Electrolysis
The increase in the production of renewable electricity offers the opportunity to transition from the usage of fossil-based hydrogen to green hydrogen. While mass production of green hydrogen by water electrolysis demands substantial freshwater resources, the abundant availability of seawater provides a promising opportunity to directly use it as an electrolyte in a water electrolyzer. However, a major challenge in seawater electrolysis is the low selectivity for oxygen evolution compared to the chlorine evolution at the anode. To address this, we proposed a strategy to boost the selectivity for oxygen evolution by hydrated electrocatalysis, in which water is itself part of the catalyst. Water molecules that are coordinately bonded to the active catalytic sites play a significant role in enhancing oxygen evolution selectivity. This approach was demonstrated with Prussian blue analogue electrocatalysts in acidified simulated seawater electrolyte using rotating ring disk electrode voltammetry. Microkinetic modeling was employed to correlate the coverage by the reactants (H2O and Cl–) with selectivity. Notably, the crystal water coverage on hydrated electrocatalysts emerged as the partial descriptor for the selectivity of the oxygen evolution reaction. To gain insights for coverage by crystal water and Cl–, the thermogravimetric analysis combined with Rietveld refinement and microkinetic Tafel analysis was performed. In a nutshell, we explored the question: if the reactant molecule (H2O) is an integral part of the catalyst, can it promote the corresponding electrochemical oxidation reaction (O2 evolution) over its competitor (Cl2 evolution)?
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


