IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在本研究中,我们首次报道了微波加速合成嘌呤和嘧啶核苷三磷酸原药的方法,γ 磷酸被芳基氧基和氨基酸酯(γ-ProTriP)掩蔽。美国 FDA 批准的两种抗癌药物氯法拉滨和吉西他滨的三磷酸原药的合成说明了这种方法的合成用途。这些新原药显示出良好的化学稳定性和大鼠血清稳定性。值得注意的是,氯法拉滨原药对一组癌细胞株具有显著的细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
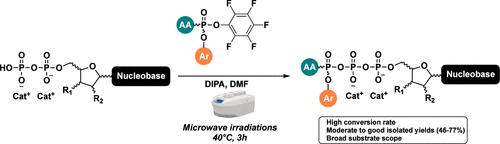
Microwave-Accelerated Synthesis of Novel Triphosphate Nucleoside Prodrugs: Expanding the Therapeutic Arsenal of Anticancer Agents
In this study, we report for the first time a microwave-accelerated synthesis of purine and pyrimidine nucleoside triphosphate prodrugs, whose γ phosphate is masked with an aryloxy moiety and an amino acid ester (γ-ProTriP). The synthetic utility of this method is illustrated by the synthesis of triphosphate prodrugs of clofarabine and gemcitabine, two FDA-approved anticancer drugs. These new prodrugs showed good chemical and rat serum stability. Remarkably the clofarabine prodrug showed significant cytotoxicity against a panel of cancer cell lines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


