N 桥接的 BODIPY 二聚体:通过吡咯和吡啶环化探索富电子和贫电子耦合极限
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
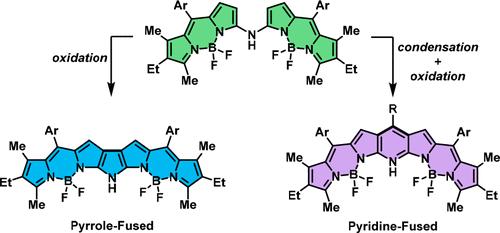
N-Bridged BODIPY Dimers: Exploring the Electron-Rich and Electron-Poor Coupling Limit via Pyrrole and Pyridine Annulation
A facile access to N-heteroaryl-fused bis-BODIPY scaffolds has been developed. A BODIPY dimer with an α,α-amine linker serves as a starting material to obtain pyrrole- and pyridine-fused BODIPYs, either by direct oxidation or by oxidative condensation with an aldehyde building block. Both species mark antipodal conjugative coupling conditions that result in distinct spectral outcomes. In stark contrast to the pyrrole fusion, pyridine-coupled species show unique panchromatic absorption profiles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


