迁移芳基交叉偶联
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
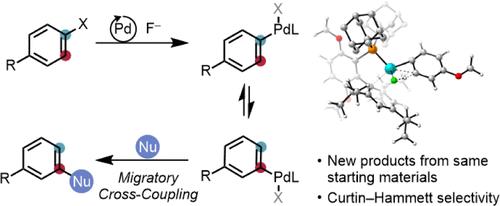
Migratory Aryl Cross-Coupling
A fundamental property of cross-coupling reactions is regiospecificity, meaning that the site of bond formation is determined by the leaving group’s location on the electrophile. Typically, achieving a different substitution pattern requires the synthesis of a new, corresponding starting-material isomer. As an alternative, we proposed the development of cross-coupling variants that would afford access to multiple structural isomers from the same coupling partners. Here, we first demonstrate that a bulky palladium catalyst can facilitate the efficient, reversible transposition of aryl halides by temporarily forming metal aryne species. Despite the nearly thermoneutral equilibrium governing this process, combining it with the gradual addition of a suitable nucleophile results in dynamic kinetic resolution of the isomeric intermediates and high yields of unconventional product isomers. The method accommodates a range of oxygen- and nitrogen-centered nucleophiles and tolerates numerous common functional groups. A Curtin–Hammett kinetic scheme is supported by computational and experimental data, providing a general mechanistic framework for extending this migratory cross-coupling concept.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


