通过环丙基碳酰阳离子化学仿生合成氮杂环戊内酯
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
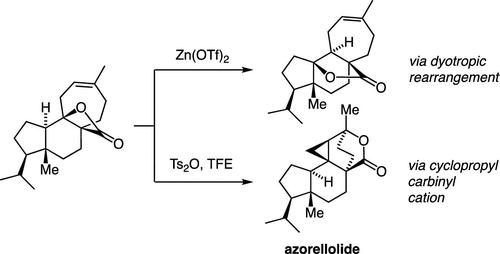
Biomimetic Synthesis of Azorellolide via Cyclopropylcarbinyl Cation Chemistry
A concise synthesis of the complex diterpene azorellolide, inspired by speculations on biosynthetic cationic cascades, is presented. The approach, guided by computation, relies on the intramolecular interception of a cyclopropylcarbinyl cation by an appended carboxylate. The successful execution of this strategy was achieved through acid-catalyzed isomerization of a β-lactone in competition with a type I dyotropic rearrangement.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


