通过烯胺-烯胺同分异构实现丙氨酸酯衍生物中 α、β-C-H 键的双重官能化:通过碎片再组装途径构建 4-喹啉酸酯骨架
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
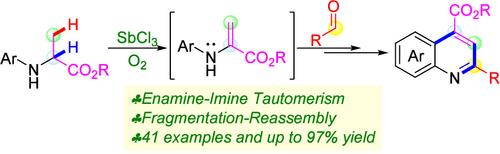
Dual Functionalization of the α,β-C–H Bonds in Alanine Ester Derivatives via Enamine–Imine Tautomerism: Construction of 4-Quinolinolate Skeletons through a Fragmentation–Reassembly Pathway
Using a SbCl3/O2 mild oxidation system, a dual functionalization of the α,β-C–H bonds in alanine ester derivatives was achieved via enamine–imine tautomerism, and a series of quinoline-4-carboxylates were synthesized through a fragmentation–reassembly pathway. The investigation of the substrate scope revealed that various functional groups were easily tolerated, highlighting that this reaction provided an efficient path for the construction of the quinoline-4-carboxylate framework.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


