分子内反马尔科夫尼科夫烯烃氢化氨基环化成顺式-2,3-二取代哌啶类化合物
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
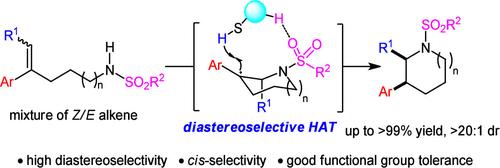
Intramolecular Anti-Markovnikov Alkene Hydroaminative Cyclization to cis-2,3-Disubstituted Piperidines
Multisubstituted piperidines are prevalent units in pharmaceuticals. Herein, a photodriven anti-Markovnikov hydroaminative cyclization of a (Z)/(E)-isomeric mixture of trisubstituted alkenes using the lactate-derived C2-symmetric arylthiol catalyst was developed for the synthesis of cis-2,3-disubstituted piperidines and azepane in high diastereoselectivity and good yields. The origin of diastereoselectivity and the observed different hydroamination rate of alkene with different configurations were elucidated by the experimental and computational investigation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


