萘上酰胺的施陶丁格裂解作用对 1,8-取代基的同位效应的影响
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
精心制备了 8-(叠氮甲基)-1-萘酸,并将其与胺偶联,得到了相应的 8-(叠氮甲基)-1-萘酰胺。8- (叠氮甲基)-1-萘酰胺与膦的施陶丁格反应生成亚氨基磷烷,亚氨基磷烷的分子内环化容易,可得到 2,3-二氢-1H-苯并[de]异喹啉-1-酮离去胺,1,8-取代基对萘的同侧效应几乎达到定量转化率。该方案具有一些优点,包括保护基团容易获得、酰胺的裂解转化率几乎达到定量、水介质、室温反应、底物范围广、官能团耐受性宽以及适合放大反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
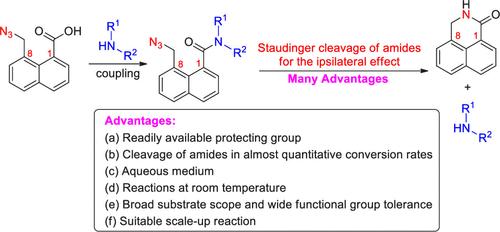
Staudinger Cleavages of Amides on Naphthalene for the Ipsilateral Effect of 1,8-Substituents
8-(Azidomethyl)-1-naphthoic acid was elaborately prepared, and its coupling with amines provided the corresponding 8-(azidomethyl)-1-naphthamides. The Staudinger reactions of 8-(azidomethyl)-1-naphthamides with phosphine produced iminophosphoranes, and easy intramolecular cyclization of the iminophosphoranes afforded 2,3-dihydro-1H-benzo[de]isoquinolin-1-one leaving amines with almost quantitative conversion rates for the ipsilateral effect of 1,8-substituents on naphthalene. The protocol exhibits some advantages, including a readily available protecting group, cleavages of amides in almost quantitative conversion rates, an aqueous medium, reactions at room temperature, a broad substrate scope, wide functional group tolerance, and suitable scale-up reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


