通过分子内非共价相互作用合成异构化双噻唑酰亚胺 (iBTzI) 受体并进行 π 延伸
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
一种异构化双噻唑亚胺(iBTzI)受体被有效合成,并通过铃木或斯蒂尔偶联反应实现了功能化。与异构化噻吩亚胺(iBTI)和双噻唑亚胺(BTzI)相比,iBTzI 的骨架更为平面。此外,iBTzI 的共轭骨架还可以通过氢键和钙键进行扩展。供体-受体-供体型(D-A-D-型)iBTzI 衍生物在溶液中显示出蓝到红的发射和增强的光致发光量子产率(ΦPLs),在发光材料中大有可为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
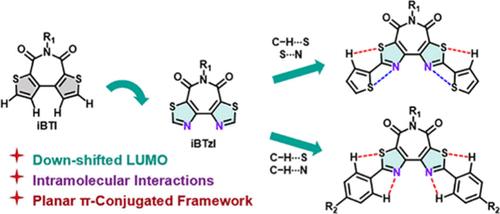
Synthesis of an Isomerized Bithiazole Imide (iBTzI) Acceptor and π-Extension via Intramolecular Noncovalent Interactions
An isomerized bithiazole imide (iBTzI) acceptor was effectively synthesized and functionalized via Suzuki or Stille coupling reactions. Compared with the isomerized bithiophene imide (iBTI) and bithiazole imide (BTzI), iBTzI has a more planar skeleton. Furthermore, the conjugated skeleton of iBTzI can be extended by hydrogen and chalcogen bonds. The donor–acceptor–donor-type (D–A–D-type) iBTzI derivatives display blue-to-red emission and enhanced photoluminescence quantum yields (ΦPLs) in solution, showing great promise in luminous materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


