有机光氧化还原催化下苯苯胺自由基α-C-H烷基化和杂芳化反应的研究
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
参与光催化的α-氨基自由基为快速构建复杂的胺结构提供了一种有吸引力的方法。本文报道了一种有机光氧化还原催化方法,用于α- c - h烷基化和苯苯胺的杂芳化,这使得在胺的α位置上引入有价值的三氟甲醇、铬罗曼酮或吡啶基序。该协议强调无金属,步骤和原子经济和广泛的底物范围(>;80例)。对照实验和电子顺磁共振波谱鉴定出α-氨基自由基来源于α-氨基C-H键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
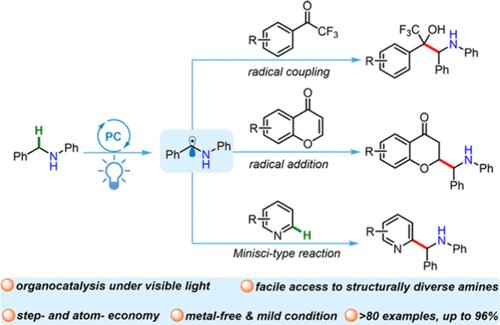
Radical α-C–H Alkylation and Heteroarylation of Benzyl Anilines Enabled by Organic Photoredox Catalysis
A photocatalysis-involved α-amino radical provides an appealing approach for rapid construction of complex amine architectures. Reported herein is an organophotoredox catalytic approach to α-C–H alkylation and heteroarylation of benzyl anilines, which enables the introduction of valuable trifluoromethyl alcohol, chromanone, or pyridine motifs at the α position of amines. This protocol highlights metal-free, step and atom economies and broad substrate scopes (>80 examples). Control experiments and electron paramagnetic resonance spectroscopy identified the α-amino radical derived from the α-amino C–H bond.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


