IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
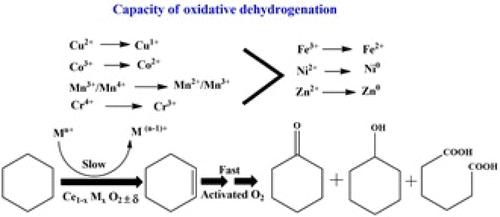
环己烷氧化成酮醇混合物(KA 油)或己二酸是一种重要的工业反应。过渡金属(TM)离子作为支撑金属氧化物催化剂的核心成分,在环己烷氧化过程中发挥着重要作用。过渡金属离子与环己烷中 C-H 键之间的相互作用机理在文献中仍未得到探讨。本研究制备了多种过渡金属(Cr、Mn、Fe、Co、Ni、Cu 和 Zn)离子取代的 CeO2 催化剂,对其进行了表征,并测试了其在以 O2 为氧化剂的环己烷氧化过程中的催化活性。计算了催化剂单位表面上环己烷转化的平均速率,以评估取代在 CeO2 晶格中的 TM 离子的内在催化反应活性。以单位表面上的铬离子数量(10% Cr/CeO2)为标准的平均速率是找到反应中最活跃的 TM 离子的参数。TM 离子取代的 CeO2 催化剂上 C-H 活化的机理途径得到了 DFT 计算的支持,表明首次形成了活性环己烯中间体。因此,我们计算了 Cu/CeO2 催化剂上环己烷和环己烯的转化率,以确定中间体的相对反应性。从平均转化率的角度来看,金属离子的反应性与掺杂过渡金属离子的电子状态有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the Intrinsic Catalytic Reactivity of Various Transition-Metal Ions Substituted in CeO2 for Cyclohexane Oxidation: A Correlation between Catalytic Activities and Electronic States of the Substituent Ions
Cyclohexane oxidation into a ketone–alcohol mixture (KA oil) or adipic acid is an industrially significant reaction. Transition metal (TM) ion, as a core component of supported metal oxide catalysts, plays a significant role in cyclohexane oxidation. The mechanism of interaction between the transition metal ion and C–H bond in cyclohexane remains unexplored in the literature. In this study, various transition metal (Cr, Mn, Fe, Co, Ni, Cu, and Zn) ion-substituted CeO2 catalysts have been prepared, characterized, and tested for catalytic activities in cyclohexane oxidation using O2 as the oxidant. The average rate of cyclohexane conversion over the unit surface of the catalysts has been calculated to evaluate the intrinsic catalytic reactivity of the TM ions substituted in the CeO2 lattice. The average rate, normalized with respect to the number of Cr ions over the unit surface (in 10% Cr/CeO2), was the parameter to find the most active TM ion for the reaction. The mechanistic pathway of C–H activation over the TM ions-substituted CeO2 catalysts has been supported by DFT calculations, indicating the formation of a reactive cyclohexene intermediate for the first time. Therefore, the rates of conversion of cyclohexane and cyclohexene over the Cu/CeO2 catalyst have been calculated to determine the relative reactivity of the intermediate. The reactivity of the metal ions, in terms of the average rate of conversion, has been correlated to the electronic state of the doped transition metal ions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


