冰结合蛋白对冰成核的分子研究:与冰成核蛋白、抗冻蛋白和非冰结合蛋白的比较研究
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
冰结合蛋白(IBPs)是一类能与冰发生特异性相互作用的特殊蛋白质。冰结合蛋白的一个亚类可以通过以非共轭方式降低冰的凝固点来抑制冰的生长,被称为抗冻蛋白(AFPs)。另一种能增强冰成核过程的亚类 IBPs 被称为冰成核蛋白(INPs)。在本研究中,我们利用分子动力学模拟诱导了冰的形成,并进一步比较了 AFP、INPs 和非冰结合蛋白(NonIBPs)的成核行为。结果发现,与 AFP 相比,INP 的初始稳定冰簇的形成更接近 T-X-T 主题,而与非冰结合蛋白相比,INP 的初始稳定冰簇的形成则最远。最大冰簇中冰分子的比例也是 INP 最高。更重要的是,在不同的独立运行中,INP 形成的冰簇的构型惊人地相似,这表明其冰结合表面在控制过程中起着至关重要的作用。AFP和NonIBP在不同的独立运行中形成的冰的构型非常不同。定量分析显示,与 AFP/NonIBP 相比,INP 更倾向于形成六方冰,而不是立方冰。在本研究使用的两种 INP 模型结构中,AlphaFold2 预测的最新模型结构比较旧的 INP 模型显示出更强的成冰能力。自由能计算也证实了这些不同类别蛋白质成核能力的类似结论。最后,在单一结构中,INP 的水组织基团比非水组织基团具有更强的冰成核能力。我们的研究结果清楚地证明了 INP 比 AFP 和其他非IBP 更能促进冰的成核。本文章由计算机程序翻译,如有差异,请以英文原文为准。
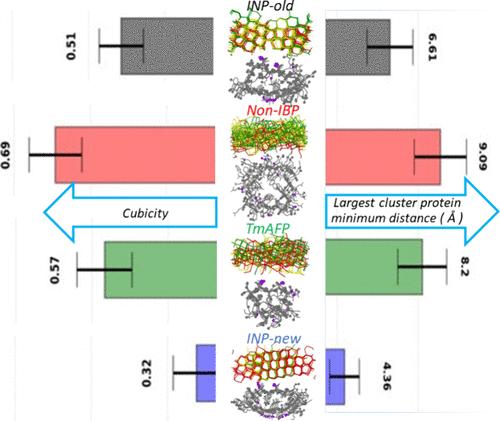
Molecular Insight of the Ice Nucleation by Ice Binding Protein: A Comparative Study With Ice Nucleating Protein, Antifreeze Protein, and Non-Ice-Binding Protein
Ice-binding proteins (IBPs) are a special class of proteins that can specifically interact with ice. A subclass of IBPs that can inhibit ice growth by depressing the freezing point of ice in a noncolligative manner are called antifreeze proteins (AFPs). The other subclass of IBPs that can enhance the ice nucleation process are called ice nucleating proteins (INPs). In this study, using molecular dynamics simulations, we induced ice formation and further compared the nucleation behaviors of AFP, INPs, and non ice binding proteins (NonIBPs). The formation of the initial stable ice cluster is found to be closer to the T-X-T motif of INP than that of AFP and it is farthest from the NonIBP. The fraction of ice molecules in the largest cluster is also found to be highest for INP. More importantly, the configurations of ice clusters formed in different independent runs are found to be strikingly similar for INP which signifies the crucial role of its ice-binding surface in controlling the process. The configurations of the formed ice in different independent runs are very dissimilar for both AFP and NonIBP. Quantitative analysis revealed that INP exhibits a stronger preference for hexagonal ice formation over its cubic counterpart compared to AFP/NonIBP. Between the two model INP structures used in this study, the latest model structure predicted by AlphaFold2 showed superior ice nucleating abilities than the older INP model. Similar conclusions about the abilities of ice nucleation by these different classes of proteins are confirmed by free energy calculation. Finally, the ice nucleating ability of the water-organizing motif of an INP is demonstrated over its nonwater-organizing motif in a single structure. Our findings provide clear evidence of INP’s ability to promote ice nucleation over AFPs and other NonIBPs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


