IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究利用第一原理密度泛函理论(DFT)和经典分子动力学(CMD)研究了多阴离子橄榄石 MgMSiO4(M = Co 和 Ni)电极材料。我们探索了这些正硅酸盐材料的结构、电子和离子动力学。我们的研究结果表明,多阴离子[SiO4]4-四面体通过形成通道,并通过其耦合的重新定向动力学促成了桨轮机制,从而促进了 Mg2+ 离子的迁移。离子扩散研究揭示了 Mg2+ 离子的低能障,表明这些材料有利于离子迁移。在这里,我们建立了弛豫时间与离子电导率之间的线性相关关系。这填补了我们对离子如何在固体晶格中独立运动或与阴离子多面体脱钩的理解空白。阳离子和阴离子的相关动力学对于控制离子传输特性至关重要。此外,在插层-插层过程中观察到的最小体积变化归功于强 Si-O 键的稳定作用,它通过感应效应削弱了 M-O 键。这些键有助于在电池工作期间保持结构稳定。MgCoSiO4 和 MgNiSiO4 的平均电压分别为 2.85 V 和 3.27 V,因此很有希望成为 MIB 阴极的候选材料。橄榄石硅酸盐是极性导体,其材料特性会影响电荷载流子。DFT 结果揭示了自旋极化的电子结构和极子的形成,为自由极子的行为提供了详细的见解。这项工作将有助于我们从原子层面了解用于高容量充电电池的聚阴离子阴极材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
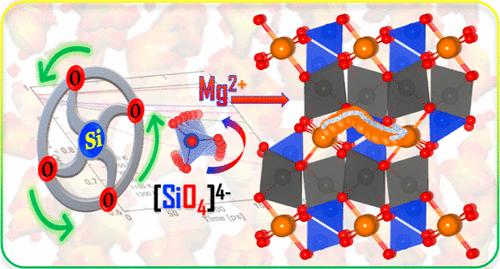
Correlated Mg-Ion and Electron Transport in Polyanionic Co and Ni Silicate Electrodes: A Paddle Wheel-like Rotation-Induced Process
This study investigates polyanionic-based olivine MgMSiO4 (M = Co and Ni) electrode materials using first-principles density functional theory (DFT) and classical molecular dynamics (CMD). The structural, electronic, and ionic dynamics of these orthosilicate materials are explored. Our findings demonstrate that polyanionic [SiO4]4– tetrahedra facilitate Mg2+ ionic mobility by forming channels and enabling the paddle-wheel mechanism through their coupled reorientation dynamics. Ionic diffusion studies reveal low-energy barriers for Mg2+ ions, indicating favorable ionic transport in these materials. Here, we establish a linear correlation between relaxation time and ionic conductivities. This can fill the gap in understanding how ions can move independently or decouple with the anionic polyhedra in the solid lattice. The correlated dynamics of cations and anions is crucial for controlling the ionic transport properties. Additionally, minimal volume changes during intercalation–deintercalation are observed, attributed to the stabilizing effect of strong Si–O bonds, which weaken M–O bonds through an inductive effect. These bonds help to maintain structural stability during battery operation. The average voltages are 2.85 V for MgCoSiO4 and 3.27 V for MgNiSiO4, making them promising candidates for MIB cathodes. Olivine silicates act as polaronic conductors, where the material properties influence charge carriers. DFT results reveal the electronic structure and polaron formation with spin polarization, providing detailed insight into the behavior of free polarons. This work will help us understand polyanionic cathode materials for high-capacity rechargeable batteries at the atomic level.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


