Cu原子系综上CO-to-Acetate电还原开关
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
电催化反应途径在很大程度上取决于催化剂的内在结构。最近,CO2/CO 电还原已成为获得 C2+ 产物的一种潜在方法,但要获得单一 C2+ 产物的高选择性却具有挑战性。在此,我们开发了一种满足电催化 CO 到醋酸盐转化所需的适当位点距离和配位环境的铜原子组合,该组合显示出出色的整体性能,在部分电流密度为 225 mA cm-2 和生成率为 2.1 mmol h-1 cm-2 的条件下,醋酸盐法拉第效率为 70.2%。此外,还能获得 91% 的单程 CO 转化率和显著的稳定性。详细的实验和理论研究证实了铜原子团在优化 C-C 偶联、稳定关键烯酮中间体(*CCO)和抑制*HOCCOH 中间体方面的显著优势,这可以将 CO 还原途径从传统金属铜位点上的乙醇/乙烯转换到铜原子团上的醋酸盐。本文章由计算机程序翻译,如有差异,请以英文原文为准。
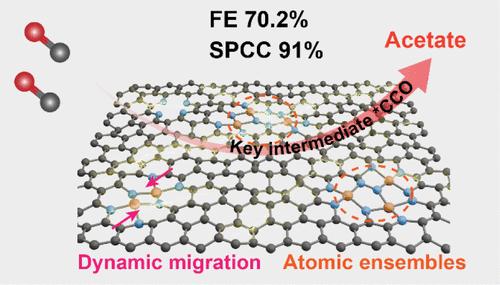
Switching CO-to-Acetate Electroreduction on Cu Atomic Ensembles
The electrocatalytic reaction pathway is highly dependent on the intrinsic structure of the catalyst. CO2/CO electroreduction has recently emerged as a potential approach for obtaining C2+ products, but it is challenging to achieve high selectivity for a single C2+ product. Herein, we develop a Cu atomic ensemble that satisfies the appropriate site distance and coordination environment required for electrocatalytic CO-to-acetate conversion, which shows outstanding overall performance with an acetate Faradaic efficiency of 70.2% with a partial current density of 225 mA cm–2 and a formation rate of 2.1 mmol h–1 cm–2. Moreover, a single-pass CO conversion rate of 91% and remarkable stability can be also obtained. Detailed experimental and theoretical investigations confirm the significant advantages of the Cu atomic ensembles in optimizing C–C coupling, stabilizing key ketene intermediate (*CCO), and inhibiting the *HOCCOH intermediate, which can switch the CO reduction pathway from the ethanol/ethylene on the conventional metallic Cu site to the acetate on the Cu atomic ensembles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


