IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
许多肽类激素在与其同源受体相互作用时会形成长的α-螺旋结构,但在未与受体结合时往往表现出灵活的构象。目前仍缺乏既能稳定长 α-螺旋结构又不破坏其与受体结合的策略,这阻碍了其生物学应用和药物开发的进展。在这里,我们介绍了一种将合理设计与文库筛选相结合的方法,以创建和鉴定一种独特的二硫定向多环肽(DDMP)支架,它可以有效地稳定 N 端可延伸的 α-螺旋,同时在二硫配对和氧化折叠方面表现出卓越的效率。随后,这种 DDMP 支架被用于稳定胰高血糖素样肽-1(GLP-1)的 α 螺旋结构,从而产生了一种强效的 GLP-1 受体(GLP-1R)激动剂,其 α 螺旋结构和蛋白水解稳定性得到了显著改善。通过在 DDMP 支架中加入外部 α-螺旋,我们可以有效地保留原生 N 端 α-螺旋结构,同时允许 C 端富二硫化物结构域发生广泛的进化,以增强与靶标的结合,DDMP 稳定化 GLP-1 (g1:Ox) 的产生就证明了这一点。g1:Ox-GLP-1R 与异三聚体 Gs 复合物的冷冻电镜结构揭示了 g1:Ox 与 GLP-1R 强效结合的分子基础。具体来说,DDMP 分子与 GLP-1R 的细胞外结构域建立了额外的相互作用,而 GLP-1 则不存在这种相互作用。因此,这项工作为治疗肽和其他肽 α-螺旋的工程设计提供了一种新颖而有效的方法,确保了 N 端和 C 端区域对于目标识别和激活仍然是必不可少的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
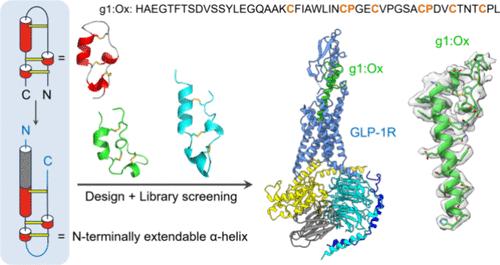
Disulfide-Directed Multicyclic Peptides with N-Terminally Extendable α-Helices for Recognition and Activation of G-Protein-Coupled Receptors
Many peptide hormones adopt long α-helical structures upon interacting with their cognate receptors but often exhibit flexible conformations when unbound. Strategies that can stabilize long α-helices without disrupting their binding to receptors are still lacking, which hinders progress in their biological applications and drug development. Here, we present an approach that combines rational design with library screening to create and identify a unique disulfide-directed multicyclic peptide (DDMP) scaffold, which could effectively stabilize N-terminally extendable α-helices while displaying exceptional efficiency in disulfide pairing and oxidative folding. This DDMP scaffold was then utilized for stabilizing the α-helical structure of glucagon-like peptide-1 (GLP-1), resulting in a potent GLP-1 receptor (GLP-1R) agonist with a significantly improved α-helicity and proteolytic stability. By incorporating external α-helices into the DDMP scaffold, we can effectively preserve the native N-terminal α-helical structures while allowing for extensive evolution of the C-terminal disulfide-rich domain for enhancing target binding, as demonstrated by the generation of the DDMP-stabilized GLP-1 (g1:Ox). The cryo-electron microscopy structure of the g1:Ox–GLP-1R in complex with heterotrimeric Gs reveals the molecular basis for the potent binding between g1:Ox and GLP-1R. Specifically, the DDMP moiety establishes additional interactions with the extracellular domain of GLP-1R, which are absent in the case of GLP-1. Thus, this work offers a novel and effective approach for engineering therapeutic peptides and other peptide α-helices, ensuring that both the N- and C-terminal regions remain essential for target recognition and activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


