MnIV(O)(μ-O)CeIV的形成和反应性:最接近光系统II的模拟物
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
了解光系统II (PS-II)中成氧络合物(OEC)的基本结构和水氧化机理,有助于发现更高效、可持续的水氧化催化剂。在这种情况下,我们提出了在[(TPA)MnII]2+(1)配合物的乙腈溶液中加入硝酸铈铵,形成[(TPA)MnII (O)(μ-O)CeIV(NO3)3]+(2)配合物(TPA =三(吡啶-2-甲基)胺)的证据。使用各种光谱技术和电喷雾电离质谱分析了这种独特的中间体(2)。值得注意的是,2与PS-II OEC中提出的MnV(O)(μ-O)CaII(OH2)的结构非常相似。值得注意的是,2与二茂铁衍生物有效反应,表明氧化还原活性CeIV结合提高了电子传递效率。此外,2展示了进行氧原子转移和氢原子提取反应的能力。这种活性[(TPA) MnV(O)(μ-O) CeIV(NO3)3]+的发现为研究OEC中MnV(O)(μ-O) CeIV(OH2)单元的结构提供了令人兴奋的机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
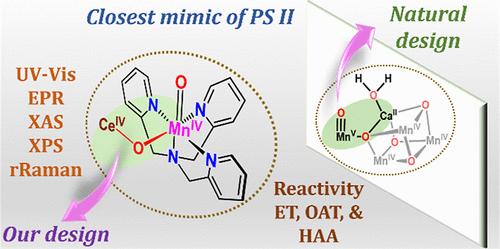
Formation and Reactivity of a MnIV(O)(μ-O)CeIV Species: A Closest Mimic of Photosystem II
Understanding the basic structure of the oxygen-evolving complex (OEC) in photosystem II (PS-II) and the water oxidation mechanism can aid in the discovery of more efficient and sustainable catalysts for water oxidation. In this context, we present evidence of the formation of a [(TPA)MnIV(O)(μ-O)CeIV(NO3)3]+ (2) complex (TPA = tris(pyridyl-2-methyl)amine) by adding aqueous ceric ammonium nitrate to an acetonitrile solution of the [(TPA)MnII]2+ (1) complex. This unique intermediate (2) was analyzed by using various spectroscopic techniques and electrospray ionization mass spectrometry. Remarkably, 2 closely mimics the structure of MnV(O)(μ-O)CaII(OH2) proposed in the OEC of PS-II. Notably, 2 reacts effectively with ferrocene derivatives, indicating that redox-active CeIV binding enhances the electron transfer efficiency. Additionally, 2 demonstrated the ability to perform oxygen atom transfer and hydrogen atom abstraction reactions. The discovery of this reactive [(TPA)MnIV(O)(μ-O)CeIV(NO3)3]+ species provides exciting opportunities for investigating the structure of the MnV(O)(μ-O)CaII(OH2) unit in the OEC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


