铱催化外消旋1,5-二醇合成哌啶和四氢异喹啉的对映收敛结构
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
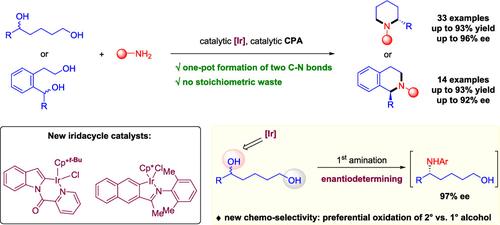
我们在本文中报告了从容易获得的外消旋 1,5 二醇一步合成有价值的对映体富集哌啶和四氢异喹啉的方法。成功的关键在于开发了新型铱环催化剂,它能以对映转化的方式在二元醇和胺之间高效地构建两个 C-N 键。机理研究发现,二元醇底物中的仲醇和伯醇在铱循环催化剂的作用下会发生有趣的优先氧化反应,这就使芳基-烷基取代醇的分子间胺化成为这种催化 N- 异环合成的对映体决定步骤。此外,还展示了这种催化方法在制备重要药物和生物活性化合物中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Iridium-Catalyzed Enantioconvergent Construction of Piperidines and Tetrahydroisoquinolines from Racemic 1,5-Diols
We report herein a one-step synthesis of valuable enantioenriched piperidines and tetrahydroisoquinolines from readily available racemic 1,5-diols. Key to the success is the development of new iridacycle catalysts that enable efficient redox-neutral construction of two C–N bonds between diols and amines in an enantioconvergent fashion. Mechanistic studies identified an intriguing preferential oxidation of secondary versus primary alcohol in the diol substrate by the iridacycle catalyst, which set a challenging intermolecular amination of aryl–alkyl-substituted alcohol as the enantiodetermining step for this catalytic N-heterocycle synthesis. Application of this catalytic method to the preparation of important drugs and bioactive compounds is also demonstrated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


