作为钴超填充抑制剂的 5-炔基嘧啶研究
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
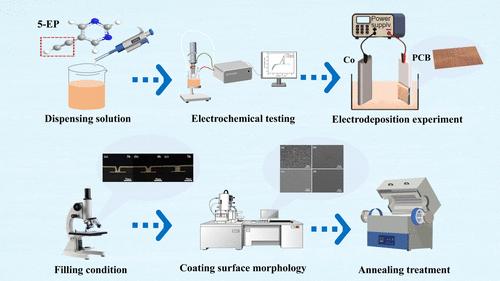
电沉积技术是高端芯片制造中实现微米/纳米级互连的关键核心技术。近年来,由于钴的电子平均自由路径小于铜,钴互连结构成为在 14 纳米以下工艺节点构建电子通路的理想选择;因此,研究钴在微纳尺度空间的电镀填充行为已成为芯片制造领域的热门话题。本文设计了一种含有新型 5-炔基嘧啶(5-EP)作为抑制剂的电镀溶液,并对其进行了电化学测试,包括循环伏安法和线性扫描伏安法。结果表明,5-EP 能显著抑制钴的电沉积。计时器显示,在含有抑制剂(5-EP)的电镀溶液中,钴原子以三维连续方式成核,而不是三维瞬时成核。原位增强拉曼光谱从光谱学角度进一步分析了电镀过程中抑制剂(5-EP)分子在钴表面的吸附情况。通过量子化学计算得到的添加剂分子的能隙值为△E = ELUMO - EHOMO = 3.302 eV,表明添加剂分子在金属界面上具有较高的反应活性和吸附强度。计算得出的分子静电位(ESP)值从最低的 -47.29 kJ/mol 到最高的 70.89 kJ/mol。较低的 ESP 值意味着较高的电子密度。分子动力学模拟计算出添加剂分子(5-EP)在 Co(100)上的吸附能为 -133.96 kcal/mol,表明 5-EP 分子具有良好的吸附能力。电镀实验表明,在电镀溶液中加入 6 ppm 的 5-EP 可实现无缺陷盲孔填充。最后,表征测试(扫描电子显微镜/原子力显微镜/X 射线衍射)还表明,抑制剂(5-EP)可有效降低钴镀层的表面粗糙度,同时促进钴沿 100 和 110 晶面优先生长。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Study of 5-Alkynylpyrimidines as Cobalt Superfilling Inhibitors
Electrodeposition technology is the key core technology for achieving micro/nanoscale interconnection in high-end chip manufacturing. Recently, cobalt interconnect structures have been the ideal choice for constructing electronic pathways in process nodes below 14 nm due to the fact that cobalt has a smaller electron mean free path compared to copper; thus, studying the electroplating filling behavior of cobalt in micronano scale space has become a hot topic in the field of chip manufacturing. In this article, an electroplating solution containing a new 5-alkynylpyrimidine (5-EP) as an inhibitor is designed for electrochemical tests, including cyclic voltammetry and linear sweep voltammetry. The results indicate that the 5-EP significantly inhibits cobalt electrodeposition. The change in the nucleation mode of cobalt in an electroplating solution with the inhibitor (5-EP) was revealed by chronoamperometry, where cobalt atoms nucleate in a three-dimensional continuous manner instead of three-dimensional instantaneous nucleation. In situ enhanced Raman spectroscopy is used to further analyze the adsorption of the inhibitor (5-EP) molecules on the cobalt surface during the electroplating process from a spectroscopic perspective. The energy gap value of the additive molecule obtained through quantum chemical calculations is △E = ELUMO – EHOMO = 3.302 eV, indicating that the additive molecule has high reactivity and higher adsorption strength at the metal interface. The calculated electrostatic potential (ESP) values of the molecule range from a minimum of −47.29 kJ/mol to a maximum of 70.89 kJ/mol. Lower ESP values imply higher electron density. Molecular dynamics simulations calculate the adsorption energy of the additive molecule (5-EP) on Co(100) to be −133.96 kcal/mol, indicating that the 5-EP molecule has good adsorption capability. Electroplating experiments reveal that the addition of 6 ppm 5-EP to the plating solution enables defect-free filling of blind holes. Finally, it is also revealed by characterization tests (scanning electron microscopy/atomic force microscopy/X-ray diffraction) that the inhibitor (5-EP) effectively reduces the surface roughness of the cobalt coating and, meantime, promotes the preferential growth of cobalt along the 100 and 110 crystal planes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


