IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
环鸟苷单磷酸(GMP)-腺苷单磷酸(AMP)合成酶(cGAS)可检测病毒或内源性 DNA,激活对感染和自身免疫性疾病的先天免疫反应。与双链 DNA 结合后,cGAS 合成 2′3′ cGMP-AMP,从而触发 I 型干扰素的产生。除了存在于细胞质和细胞核中,cGAS 还存在于质膜上,但其意义尚不清楚。在此,我们报告了 cGAS 与人和小鼠巨噬细胞质膜上的肌球蛋白 1F(MYO1F)的关联。在病毒感染过程中,脾脏相关酪氨酸激酶(SYK)将 MYO1F 磷酸化,促进了赖氨酸乙酰转移酶 2A (KAT2A)的招募,KAT2A 将 cGAS 的赖氨酸残基 421、292 和 131 乙酰化,这对其活化至关重要。此外,膜定位的 cGAS 对信号激活和 I 型干扰素的产生至关重要,病毒细胞融合触发的信号激活和 I 型干扰素的产生是由细胞器释放 Mn2+ 引起的。我们的研究结果突显了 MYO1F 介导的 cGAS 定位对其在应对病毒感染时完全激活的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
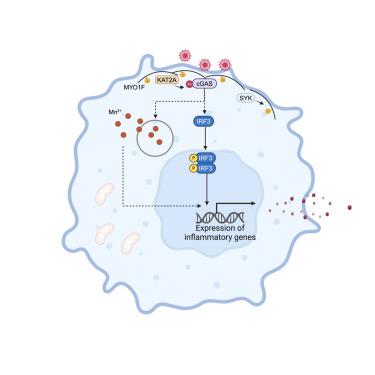
MYO1F positions cGAS on the plasma membrane to ensure full and functional signaling
Cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS) detects viral or endogenous DNA, activating the innate immune response to infections and autoimmune diseases. Upon binding to double-stranded DNA, cGAS synthesizes 2′3′ cGMP-AMP, which triggers type I interferon production. Besides its presence in the cytosol and nucleus, cGAS is found at the plasma membrane, although its significance remains unclear. Here, we report that cGAS associates with myosin 1F (MYO1F) at the plasma membrane of human and mouse macrophages. During viral infection, phosphorylation of MYO1F by spleen-associated tyrosine kinase (SYK) facilitates the recruitment of lysine acetyltransferase 2A (KAT2A), which acetylates cGAS at lysine residues 421, 292, and 131, essential for its activation. Moreover, membrane-localized cGAS is crucial for signaling activation and type I interferon production triggered by virus-cell fusion due to Mn2+ release from organelles. Our results highlight the importance of MYO1F-mediated cGAS localization for its full activation in response to viral infection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


