通过 Cu(II)-Catalyzed Amination 和 N-氧化物与邻烷基苯胺之间的腈化反应合成喹啉-吲哚杂化物
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文介绍了在铜(II)催化剂和双齿 2,2′-联吡啶配体的共同作用下,通过(异)喹啉 N-氧化物与邻炔基苯胺反应合成(异)喹啉-吲哚杂化物的方法。通过对合成产物进行位点选择性官能化,证明了这种方法的实用性。通过一系列机理研究,阐明了位点选择性胺化然后形成环状吲哚的合理反应途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
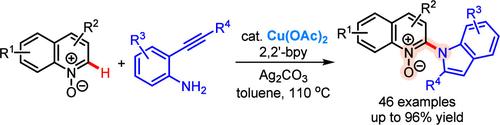
Synthesis of Quinoline–Indole Hybrids through Cu(II)-Catalyzed Amination and Annulation between N-Oxides and o-Alkynylanilines
The synthesis of (iso)quinoline-indole hybrids by reacting (iso)quinoline N-oxides with o-alkynylanilines in the presence of a combination of copper(II) catalyst and a bidentate 2,2′-bipyridine ligand is described. The utility of this method was demonstrated through site-selective functionalization of the synthesized products. A plausible reaction pathway for site-selective amination followed by annulative indole formation was elucidated by a series of mechanistic investigations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


