溶解度-平衡辅助的动力学解析聚合反应,向含有轴向手性的异构聚酯转化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
对聚合物立体化学的高度控制有助于对材料特性进行微调,但这仍然是合成聚合物化学领域的一项艰巨挑战。在此,我们制备了一类新型盐基钇催化剂,该催化剂含有轴向手性二萘基,可用于rac-Me-DBO 的轴向立体控制聚合。(S)-Y3含有较膨松的二萘基单元,通过动力学解析聚合实现了适度的等选择性,可得到Pm高达0.80的P(Me-BDO)。值得注意的是,利用溶解度平衡来保持溶液状态中两对映纯单体对的浓度恒定,有助于提高聚合的等选择性,并产生 Pm 值高达 0.93 的异构 P(Me-DBO)产品。详细的机理研究支持了我们的溶解度-平衡移动假设。这种溶解度-平衡辅助动力学解析聚合策略有望成为一种通用平台,无需重新设计催化剂即可改善立体控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
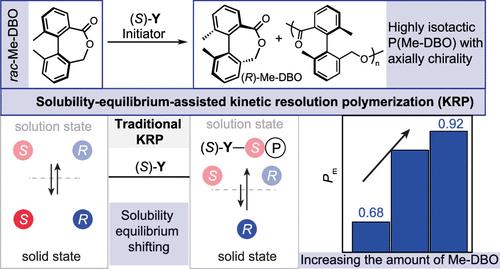
Solubility-Equilibrium-Assisted Kinetic Resolution Polymerization toward Isotactic Polyesters Containing Axial Chirality
High-level control over polymer stereochemistry leverages the fine-tuning of material properties, but it is still a formidable challenge in synthetic polymer chemistry. Herein we prepared a new class of salph yttrium catalysts bearing axially chiral binaphthyl moieties for axially stereocontrolled polymerization of rac-Me-DBO. (S)-Y3-bearing bulkier binaphthyl units accomplished moderate isoselectivity via kinetic resolution polymerization, affording P(Me-BDO) with a Pm of up to 0.80. Remarkably, exploiting the solubility equilibrium to maintain a constant for the concentration of two enantiopure monomer pairs in the solution state contributed to a boost in polymerization isoselectivity and furnished isotactic P(Me-DBO) products with a Pm of up to 0.93. Detailed mechanistic investigations supported our solubility-equilibrium shifting hypothesis. This solubility-equilibrium-assisted kinetic resolution polymerization strategy was expected to become a versatile platform to improve stereocontrol without de novo catalyst design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


