达到+1和零形式氧化态的镱配合物的多电子氧化还原化学
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
镧系元素的氧化还原反应性仍然局限于单电子转移反应,因为它们无法进入广泛的氧化态。在这里,我们展示了利用三方三(硅氧)炔氧化还原活性配体实现镱的多电子还原化学反应,这种配体可以在炔锚中存储两个电子。三(硅氧烷)炔三元配体的镱(III)复合物通过金属中心还原生成镱(II)类似物。随后的两次还原主要发生在配体上,配体框架得以保留,并在形式+1和零氧化态形成类似的镱络合物。我们分离出了四种不同氧化态的镱络合物,对其进行了晶体学和光谱学表征,并通过 DFT 研究确认了它们的电子结构。反应性研究表明,"Yb(I) "配合物可以将两个电子转移到有机叠氮化物上,同时保留其分子结构,形成高活性的亚氨基中间体,这提供了一个在单一镧系元素中心进行双电子转移而不涉及进入 +4 氧化态的罕见实例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
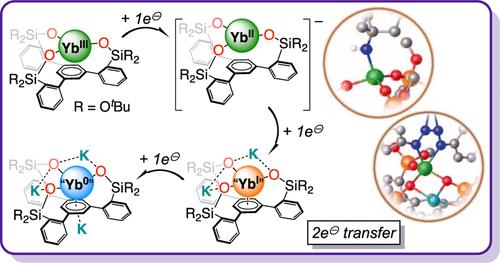
Multielectron Redox Chemistry of Ytterbium Complexes Reaching the +1 and Zero Formal Oxidation States
Lanthanide redox reactivity remains limited to one-electron transfer reactions due to their inability to access a broad range of oxidation states. Here, we show that multielectron reductive chemistry is achieved for ytterbium by using the tripodal tris(siloxide)arene redox-active ligand, which can store two electrons in the arene anchor. Reduction of the Yb(III) complex of the tris(siloxide)arene tripodal ligand affords the Yb(II) analogue by metal-centered reduction. Two subsequent reduction events occur mainly at the ligand with retention of the ligand framework and formation of analogous complexes of Yb in the formal +1 and zero oxidation states. Four complexes of Yb in four different oxidation states were isolated, crystallographically and spectroscopically characterized, and their electronic structure was confirmed by DFT studies. Reactivity studies show that the “Yb(I)” complex can transfer two electrons to organic azides, with retention of its molecular structure, to form highly reactive imido intermediates, providing a rare example of a two-electron transfer at a single lanthanide center that does not involve accessing the +4 oxidation state.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


