用于激活银屑病腺苷 A3 受体的光开关式二氮嗪衍生物
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
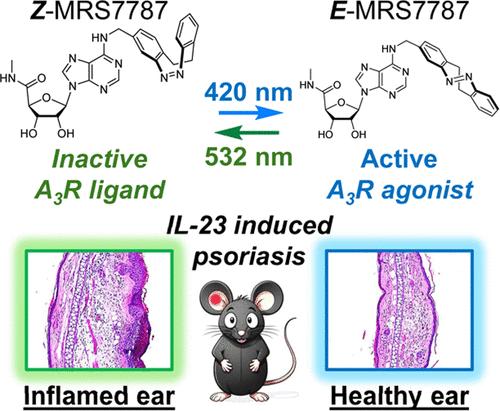
在药物中加入可光异构的分子,可以在活性和非活性构型之间实现快速、可逆的光依赖性切换。在这里,我们开发了一种可光开关的腺苷 A3 受体(A3R)激动剂,通过对银屑病动物模型进行非侵入性局部皮肤照射,对这种 G 蛋白偶联受体进行光控制。它是通过将腺苷-5′-甲基尿酰胺分子共价键合到重氮吖啶光色团上实现的,重氮吖啶光色团的奇异光开关特性促进了热稳定、无生物活性的 Z 激动剂形式与光诱导、有药理活性的 E 构型之间的反复相互转换。因此,我们的光开关激动剂可以在体外和体内精确调节 A3R 的功能,从而对小鼠皮肤病变产生明显的光控药物治疗效果。这一突破不仅证明了二氮嗪光开关在体内光药理学方面的潜力,还为开发治疗皮肤相关疾病的新策略铺平了道路,这些疾病需要局部和时间可控的药物作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Photoswitchable Diazocine Derivative for Adenosine A3 Receptor Activation in Psoriasis
Incorporating photoisomerizable moieties within drugs offers the possibility of rapid and reversible light-dependent switching between active and inactive configurations. Here, we developed a photoswitchable adenosine A3 receptor (A3R) agonist that confers optical control on this G protein-coupled receptor through noninvasive topical skin irradiation in an animal model of psoriasis. This was achieved by covalently bonding an adenosine-5′-methyluronamide moiety to a diazocine photochrome, whose singular photoswitching properties facilitated repeated interconversion between a thermally stable, biologically inactive Z agonist form and a photoinduced, pharmacologically active E configuration. As a result, our photoswitchable agonist allowed the precise modulation of A3R function both in vitro and in vivo, which led to a clear light-controlled pharmacotherapeutic effect on mouse skin lesions. This breakthrough not only demonstrates the potential of diazocine photoswitches for in vivo photopharmacology but also paves the way for the development of new strategies for skin-related diseases that require localized and temporally controlled drug action.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


