经典霍奇金淋巴瘤肿瘤微环境中的 T 细胞多样性和血源性 T 细胞排斥作用
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
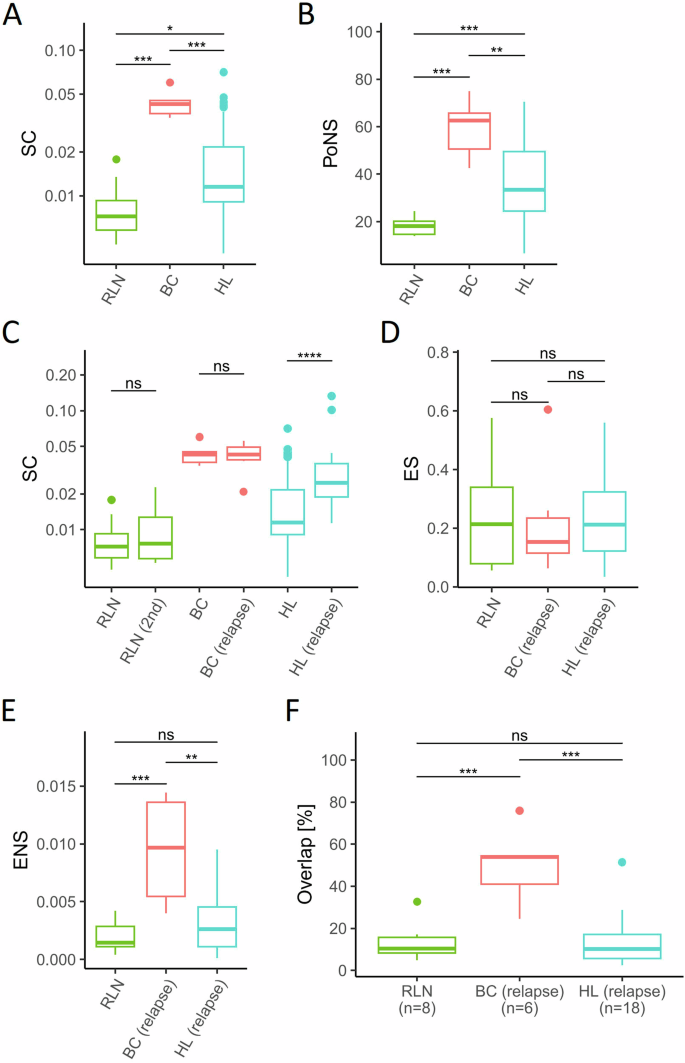
经典霍奇金淋巴瘤(HL)的肿瘤微环境(TME)中含有大量免疫细胞,只有少数肿瘤性霍奇金细胞和里德-斯特恩伯格细胞(HRSC)。我们分析了T细胞受体(TCR)谱系,以检测TME和血液中T细胞的扩增情况。与实体瘤组织不同,HL的TME中的T细胞在初诊时高度多克隆,在抗PD1免疫检查点阻断(ICB)期间仅表现出轻微的克隆扩增。在复发和 ICB 期间,实体瘤 TME 中的预扩增 T 细胞群会增加,但在 HL 中增加的程度要小得多。相比之下,HL 患者外周血中的 T 细胞群的克隆度高于健康对照组,达到了与实体癌相当的克隆度水平。然而,HL 患者的预扩增血液 T 细胞在 ICB 期间仅表现出轻微的额外克隆扩增。此外,血液中的 T 细胞在 HL 的 TME 中的重新填充程度与实体瘤中观察到的不同。因此,HL 的 TME 中的 T 细胞谱似乎是独特的,其克隆 T 细胞含量相对较低,外周血中排除了克隆扩增的 T 细胞。将克隆扩增的肿瘤特异性T细胞排除在TME之外可能是HL免疫逃避的一种新机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。

T-cell diversity and exclusion of blood-derived T-cells in the tumor microenvironment of classical Hodgkin Lymphoma
The Tumor Microenvironment (TME) in classical Hodgkin Lymphoma (HL) contains abundant immune cells and only few neoplastic Hodgkin and Reed-Sternberg cells (HRSC). We analyzed the T-cell receptor (TCR) repertoire to detect T-cell expansion in the TME and blood. In contrast to solid cancer tissue, T-cells in the TME of HL are highly polyclonal at first diagnosis and show only minor clonal expansion during anti-PD1 immune checkpoint blockade (ICB). At relapse and during ICB, pre-amplified T-cell populations increase in the TME of solid cancers but to a much lesser extent in HL. In contrast, T-cell populations in the peripheral blood of HL patients display higher clonality than healthy controls reaching clonality levels comparable to solid cancer. However, pre-amplified blood T-cells in HL patients show only minor additional clonal expansion during ICB. Moreover, blood-derived T-cells do not repopulate the TME of HL to the same extent as observed in solid cancers. Thus, the T-cell repertoire in the TME of HL appears unique by a relatively low clonal T-cell content and the exclusion of clonally expanded T-cells from the peripheral blood. Exclusion of clonally expanded tumor-specific T-cells from the TME may present a novel mechanism of immune evasion in HL.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


