KMF3钙钛矿八面体单元中m位电荷对称的破坏导致氨中硝酸盐的电化学还原增强。
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
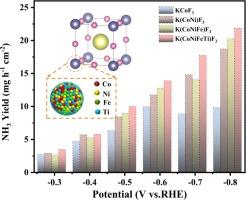
NO3-电化学生产NH3为解决NO3-过剩的环境问题提供了一个解决方案,但缺乏高效、敏感、高NH3产率和法拉第效率(FE)的电催化剂。本文制备了中熵钙钛矿氟化物(KMF3, M = Co/Ni/Fe/Ti)作为NO3-还原反应(NO3- rr)生成NH3的高效电催化剂。通过在m位引入各种过渡金属,调整m位八面体单元上的电荷分布,增加KMF3的无序性,从而获得具有高本征NO3-RR性能的优化电子结构。不同过渡金属在m位的存在促进了电子转移,抑制了Co 1s-3d的转变,增强了CoF键,导致晶体对称性降低。此外,由于价态、电负性和离子半径的差异,使得配位数减少,导致局部扭曲配位环境的产生。这有利于催化剂吸附和NO键断裂。结果表明,KMF3的NH3产率为13.9 mg h-1 cm-2, NH3 FE接近100%。所研制的中熵KMF3电催化剂具有回收硝态氮废水中氨的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The breaking of charge symmetry at the M-site in octahedral units of KMF3 perovskite induces enhanced electrochemical nitrate reduction in ammonia
Electrochemical production of NH3 from NO3− offers a solution to the environmental issue of excess NO3− but is challenged by the lack of efficient, sensitive electrocatalysts with high NH3 yield rate and Faraday efficiency (FE). Herein, a medium-entropy perovskite fluoride (KMF3, M = Co/Ni/Fe/Ti) was prepared as efficient electrocatalysts to produce NH3 via the NO3− reduction reaction (NO3−RR). By introducing various transition metals at the M-sites, the charge distribution at the M-site octahedral units was adjusted to increase the disorder of KMF3, resulting in an optimized electronic structure with high intrinsic NO3−RR performance. The presence of different transition metals at the M-sites promoted electron transfer, which inhibited the Co 1s-3d transition and strengthened the Co![]() F bond, leading to a decrease in crystal symmetry. Furthermore, owing to differences in valence state, electronegativity, and ionic radius, the coordination number was reduced, inducing the generation of a local twisted coordination environment. This was conducive to catalyst adsorption and the breaking of N
F bond, leading to a decrease in crystal symmetry. Furthermore, owing to differences in valence state, electronegativity, and ionic radius, the coordination number was reduced, inducing the generation of a local twisted coordination environment. This was conducive to catalyst adsorption and the breaking of N![]() O bonds. As a result, the KMF3 exhibited a high NH3 yield rate of 13.9 mg h−1 cm−2 with an NH3 FE close to 100 %. The developed medium-entropy KMF3 electrocatalysts have the potential to recover NH3 from nitrate wastewater.
O bonds. As a result, the KMF3 exhibited a high NH3 yield rate of 13.9 mg h−1 cm−2 with an NH3 FE close to 100 %. The developed medium-entropy KMF3 electrocatalysts have the potential to recover NH3 from nitrate wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


