连续生成C(sp3) -C (sp2)键的Ni(I)/Ni(III)选择性工艺
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
镍催化的多组分交叉偶联已经成为一种有效地从多种有机卤化物中构建复杂分子结构的强大策略。尽管具有潜力,但在一次操作中选择性地形成多个化学键,特别是在交叉亲电偶联催化领域,仍然是一个重大挑战。在这项研究中,我们开发了一个连续的开壳还原性Ni催化,使两个立体电子相似的C(sp2) -I反应物与亚甲基亲电试剂结合形成两个双生C(sp3) -C (sp2)键。使用锆氮吡啶和单质Mg0作为还原剂,该方案具有广泛的适用性,适用于广泛的(杂)芳香族、烯基和卤化物,允许快速组装具有优异官能团耐受性的医学相关支架。进一步的动力学研究表明,在镍催化中,锆氮嘧啶介导的氧化还原-金属转化过程促进了双重“顺序还原”催化过程。值得注意的是,Ni(I) -I在C(sp2) -I键上的协同氧化加成,以及C(sp2)-Ni(I)类开壳体在各种C(sp3)亲电试剂之间的卤化物原子抽离,都具有高选择性。在XEC中使用具有异常高反应活性的不对称亚甲基亲电试剂导致在反应开始时苯基或烯丙基亲电试剂中间体的快速积累,从而精细地控制偶联序列。本文章由计算机程序翻译,如有差异,请以英文原文为准。
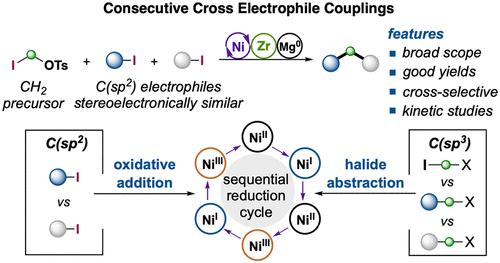
Selective Ni(I)/Ni(III) Process for Consecutive Geminal C(sp3)–C(sp2) Bond Formation
Ni-catalyzed multicomponent cross-couplings have emerged as a powerful strategy for efficiently constructing complex molecular architectures from a diverse array of organic halides. Despite its potential, selectively forming multiple chemical bonds in a single operation, particularly in the realm of cross-electrophile coupling catalysis, remains a significant challenge. In this study, we have developed a consecutive open-shell reductive Ni catalysis, enabling the formation of two geminal C(sp3)–C(sp2) bonds from two stereoelectronically similar C(sp2)–I reactants in conjunction with a methylene electrophile. Using zirconaaziridine and elemental Mg0 as reductants, this protocol exhibits broad applicability across a wide range of (hetero)aromatic, alkenyl, and glycal halides, allowing for the rapid assembly of medicinally relevant scaffolds with excellent functional group tolerance. Further kinetic studies suggest a dual “sequential reduction” catalytic process facilitated by a zirconaaziridine-mediated redox-transmetalation process in Ni catalysis. Notably, the concerted oxidative addition of Ni(I)–I across a C(sp2)–I bond, as well as the halide atom abstraction among various C(sp3) electrophiles by an open-shell C(sp2)-Ni(I) species, can proceed with high selectivity. The use of an unsymmetrical methylene electrophile with exceptionally high reactivity in XEC resulted in the rapid accumulation of a benzylic or allylic electrophile intermediate at the outset of reaction, thereby finely controlling the coupling sequence.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


