利用氧乙烷和氮杂啶磺酰氟为药物发现创造机会
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
四元杂环(如氧杂环丁烷和氮杂环丁烷)因其体积小、极性强,且具有显著影响药物分子理化性质的潜力,成为药物化学中极具吸引力的新兴设计选择。这些衍生物的制备难度很大,尤其是以不同的方式制备,严重限制了它们与其他具有重要药物和生物意义的基团的结合。因此,人们亟需温和有效的合成策略来获得新的氧杂环丁烷和氮杂环丁烷衍生物和分子支架。在此,我们报告了氧杂环丁烷磺酰氟(OSFs)和氮杂环丁烷磺酰氟(ASFs)的开发和使用情况,它们在不寻常的脱氟磺酰化反应途径(deFS)中可作为碳原子的前体。小环磺酰氟在温和的热条件(60 °C)下被活化,生成的反应中间体可与多种亲核物偶联。制备出氧杂环丁烷和氮杂环丁烷杂环化合物、-亚磺酰亚胺和-膦酸盐衍生物,其中有几种没有类似的羰基类似物,为药物发现提供了新的化学主题和设计元素。另外,在阴离子条件下,SuFEx 途径可获得氧杂环丁烷-硫(VI)衍生物。我们通过 11 种药物类似物的合成,展示了新型 OSF 和 ASF 试剂的合成效用,显示了它们后续多样化的潜力,并可方便地纳入药物化学计划。此外,我们还建议将 OSF 和 ASF 试剂用作连接基团,并展示了适合常见共轭反应的悬垂基团的加入。与 E3 连接酶招募剂(如泊马度胺和相关衍生物)的富有成效的脱FS 反应提供了新的降解剂基团和潜在的 PROTAC 连接剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
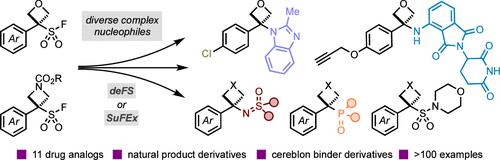
Harnessing Oxetane and Azetidine Sulfonyl Fluorides for Opportunities in Drug Discovery
Four-membered heterocycles such as oxetanes and azetidines represent attractive and emergent design options in medicinal chemistry due to their small and polar nature and potential to significantly impact the physiochemical properties of drug molecules. The challenging preparation of these derivatives, especially in a divergent manner, has severely limited their combination with other medicinally and biologically important groups. Consequently, there is a substantial demand for mild and effective synthetic strategies to access new oxetane and azetidine derivatives and molecular scaffolds. Here, we report the development and use of oxetane sulfonyl fluorides (OSFs) and azetidine sulfonyl fluorides (ASFs), which behave as precursors to carbocations in an unusual defluorosulfonylation reaction pathway (deFS). The small-ring sulfonyl fluorides are activated under mild thermal conditions (60 °C), and the generated reactive intermediates couple with a broad range of nucleophiles. Oxetane and azetidine heterocyclic, -sulfoximine, and -phosphonate derivatives are prepared, several of which do not have comparable carbonyl analogs, providing new chemical motifs and design elements for drug discovery. Alternatively, a SuFEx pathway under anionic conditions accesses oxetane-sulfur(VI) derivatives. We demonstrate the synthetic utility of novel OSF and ASF reagents through the synthesis of 11 drug analogs, showcasing their potential for subsequent diversification and facile inclusion into medicinal chemistry programs. Moreover, we propose the application of the OSF and ASF reagents as linker motifs and demonstrate the incorporation of pendant groups suitable for common conjugation reactions. Productive deFS reactions with E3 ligase recruiters such as pomalidomide and related derivatives provide new degrader motifs and potential PROTAC linkers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


