通过高分辨率电子显微镜揭示手性无机纳米晶体的生长机制
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
手性无机纳米材料由于其引人注目的手性依赖性能而引起了广泛的关注。然而,对其生长机理的实验研究和原子水平的证据缺乏。本文以氧化碲为无机源,水合肼为还原剂,在碱溶液中合成了高结晶手性碲纳米线。利用高分辨率电子显微镜和电子衍射分析了核和晶域的演化过程,证明了非经典的生长路径,即从单体到团簇的纳米线再到纳米晶体。此外,在不同阶段使用手性诱导剂d/l-青霉胺,研究其对两种具有不同手性空间群的对构结构的偏置影响。在手性磷酸铽纳米线的合成中也发现了类似的非经典生长机制,证明了手性无机纳米材料中常见的生长现象。这项工作为手性纳米材料的形成提供了新的见解,有利于进一步可控地合成各种手性纳米材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
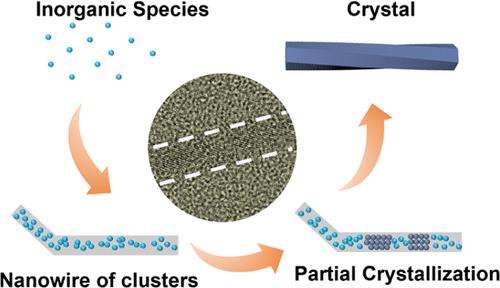
Unraveling the Growth Mechanism of Chiral Inorganic Nanocrystals via High-Resolution Electron Microscopy
Chiral inorganic nanomaterials have attracted broad interest due to their intriguing chirality-dependent performances. However, there is a lack of experimental studies and atomic-level evidence on their growth mechanism. Herein, high-crystalline chiral tellurium nanowires were synthesized in an alkali solution by using tellurium oxide as an inorganic source and hydrazine hydrate as a reductant. The evolution of the nucleus and crystalline domains was manifested using high-resolution electron microscopy and electron diffraction, demonstrating a nonclassical growth path, that is, from monomers to nanowires of clusters and then nanocrystals. Furthermore, chiral inducers, d/l-penicillamine, were used at different stages to study their effects on the bias of two enantiomorphic structures with different chiral space groups. A similar nonclassical growth mechanism was also found in the synthesis of chiral terbium phosphate nanowires, demonstrating a common growth phenomenon in chiral inorganic nanomaterials. This work provides novel insights into the formation of chiral nanomaterials, benefiting the further controllable synthesis of various chiral nanomaterials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


