等离子体-电化学合成氨过程中混氧反应途径的控制
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
众所周知,二氮(N2)的电化学活化具有挑战性,通常产生非常低的氨(NH3)收率。在这项研究中,我们提出了一个连续流等离子体电化学反应器系统,用于将空气中的氮直接转化为氨。在我们的系统中,氮分子首先在等离子体反应器中转化为氮氧化物的混合物,然后将其送入电化学反应器。为了选择性地将生成的NOx物种转化为NH3,我们采用图论方法结合第一性原理计算,全面列举了从n2到NH3的所有可能途径,精确定位了关键中间体(NH2*和NO*)。然后设计了一系列双金属催化剂,以实现nox - nh3途径中限制中间体的最佳吸附和转化。使用优化的CuPd泡沫催化剂,我们证明了在施加电流为2a的情况下,氨的产率为81.2 mg h - 1 cm-2,稳定性超过1000小时。本文章由计算机程序翻译,如有差异,请以英文原文为准。
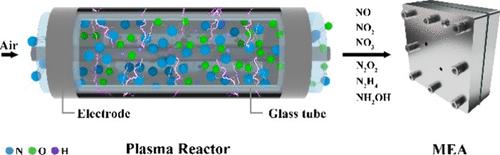
Controlling the Reaction Pathways of Mixed NOxHy Reactants in Plasma-Electrochemical Ammonia Synthesis
Electrochemical activation of dinitrogen (N2) is notoriously challenging, typically yielding very low ammonia (NH3) production rates. In this study, we present a continuous flow plasma-electrochemical reactor system for the direct conversion of nitrogen from air into ammonia. In our system, nitrogen molecules are first converted into a mixture of NOx species in the plasma reactor, which are then fed into an electrochemical reactor. To selectively convert the generated NOx species into NH3, we employed a graph theory approach combined with first-principles calculations to comprehensively enumerate all possible pathways from N2-to-NH3, pinpointing key intermediates (NH2* and NO*). A series of bimetallic catalysts was then designed to target the optimal adsorption and conversion of the limiting intermediate in the NOx-to-NH3 pathway. Using an optimized CuPd foam catalyst, we demonstrated an ammonia production rate of 81.2 mg h–1 cm–2 with stability over 1000 h at an applied current of 2 A.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


