由脱芳环氧化物-吲哚环化引发的中断Plancher重排:吲哚的正式非polung反应性
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们在此报告了偶然发现的由hfip促进的脱芳香环氧化物-吲哚环化引发的中断的Plancher重排,揭开了吲哚正式C3掺杂反应性的新蓝图。这种快速生成复杂的级联过程为高产量、全区域和非均匀控制的新型熔融桥接吲哚铺平了道路。该反应适用于起始原料中各种取代基。本文章由计算机程序翻译,如有差异,请以英文原文为准。
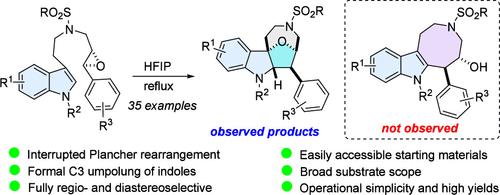
Interrupted Plancher Rearrangement Initiated by Dearomative Epoxide–Indole Cyclization: Formal Umpolung Reactivity of Indoles
We herein report the serendipitous discovery of the interrupted Plancher rearrangement initiated by an HFIP-promoted dearomative epoxide–indole cyclization, unlocking a new blueprint to the formal C3 umpolung reactivity of indoles. This rapid complexity generating cascade process paves the way toward a new class of fused–bridged indolines in high yields and under full regio- and diastereocontrol. The reaction is amenable to a wide range of substituents in the starting materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


