pt基金属间化合物电化学稳定性的理论研究
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
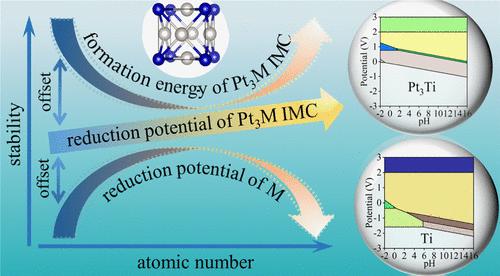
铂基金属间化合物(IMCs)具有有序的组成金属原子阵列,在能量转换应用中引起了越来越多的关注,但对其电化学稳定性的深入了解仍然缺失。在这项研究中,我们采用密度泛函理论(DFT)计算与实验热力学数据相结合的方法,探讨了影响 25 种 L12-Pt3M IMC 在水介质中相稳定性的关键因素。我们的研究表明,L12-Pt3M IMC 在还原电位下保持其金属相,但在氧化条件下会溶解为铂和锰衍生的氧化物或溶解物。我们发现 M 金属的还原电位和 IMC 的形成能量是影响 L12-Pt3M IMC 水稳定性的两个关键因素。有趣的是,在金属间结构中加入早期金属或晚期金属都能扩大 L12-Pt3M IMC 金属相的潜在稳定范围,这可归因于由 d-d 轨道杂化介导的 Pt-M 准共价结构网络的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Theoretical Insights into the Electrochemical Stability of Pt-Based Intermetallic Compounds
Pt-based intermetallic compounds (IMCs) with ordered atomic arrays of constituent metals have drawn increasing attention for energy conversion applications, but a deep understanding of their electrochemical stability is still missing. In this work, using a scheme combining density functional theory (DFT) calculations with experimental thermodynamic data, we explore key factors affecting the phase stability of 25 L12-Pt3M IMCs in aqueous media. We show that L12-Pt3M IMCs maintain their metal phase at reductive potentials but will dissolve into Pt- and M-derived oxides or dissolved species at oxidative conditions. We identify the reduction potential of the M metal and the formation energy of the IMC as the two key factors affecting the aqueous stability of L12-Pt3M IMCs. Interestingly, including either early or late metals into the intermetallic structure can both enlarge the potential range for stabilizing the metal phase of L12-Pt3M IMCs, which can be attributed to the formation of Pt-M quasi-covalent structural networks mediated by the d-d orbital hybridizations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


