碳离子共聚诱导结晶合成交替结晶共聚物的取代基调控
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
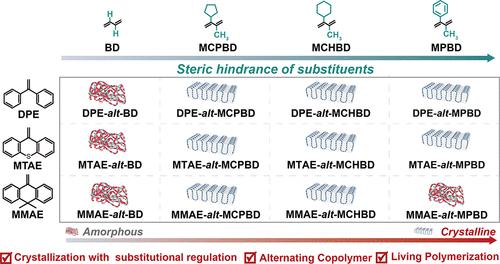
我们之前开发了一种名为 "碳阴离子共聚诱导结晶(CCPIC)"的方法,通过调整共聚物的组成而不是增加某种立体化学结构的含量来制备交替结晶共聚物。在这项工作中,通过调节 CCPIC 中使用的共轭二烯的取代基结构,开发出了八种新型交替结晶共聚物。在纯净条件下,这些共轭二烯可与 1,1-二苯基乙烯(DPE)及其衍生物(9-亚甲基-9H-硫杂蒽,MTAE;9,9-二甲基-10-亚甲基-9,10-二氢蒽,MMAE)交替生长。MCPBD)和具有更大立体阻碍的 2-环己基/3-CH3(2-环己基-3-甲基-1,3-丁二烯,MCHBD),交替共聚物 DPE/MTAE/MMAE-alt-MCPBD/MCHBD 具有结晶特性。DPE(不含桥结构)-alt-MCPBD/MCHBD 的结晶度较低,而 MMAE(含两个甲基取代基的桥结构)-alt-MCPBD/MCHBD 的结晶度则随着取代基大小的增加而降低。这表明 DPE 及其衍生物的结构会影响这些交替聚合物的聚集结构。有趣的是,DPE 及其衍生物(MTAE 和 MMAE)的聚合物链中没有手性碳原子或立体异构体。聚合物从无定形到结晶的转变是通过改变用于交替共聚的二烯和 DPE 衍生物的取代基结构实现的。因此,这些结晶聚合物在 CCPIC 中的结晶可归因于共聚单体结构带来的立体阻碍效应,而不仅仅是链中单体单元的立体规整性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Substituent Regulation of Alternating Crystalline Copolymers Synthesized via Carbanionic Copolymerization-Induced Crystallization
We previously developed a method called carbanionic copolymerization-induced crystallization (CCPIC) to prepare alternating crystalline copolymers by adjusting the copolymer composition rather than by increasing the content of a certain stereochemical structure. In this work, eight new alternating crystalline copolymers were developed by regulating the substituent structure of conjugated dienes used in CCPIC. These types of conjugated dienes can achieve alternating growth with 1,1-diphenylethylene (DPE) and its derivatives (9-methylene-9H-thioxanthene, MTAE; 9,9-dimethyl-10-methylene-9,10-dihydroanthracene, MMAE) under neat conditions. When the substituents at the 2- or 3-position of the dienes change from 2-H/3-H (butadiene, BD) to 2-cyclopentyl/3-CH3 (2-cyclopentyl-3-methyl-1,3-butadiene, MCPBD) and 2-cyclohexyl/3-CH3 (2-cyclohexyl-3-methyl-1,3-butadiene, MCHBD) with greater steric hindrance, the alternating copolymers DPE/MTAE/MMAE-alt-MCPBD/MCHBD exhibit crystallization characteristics. DPE (without a bridge structure)-alt-MCPBD/MCHBD exhibits low crystallinity, and the crystallinity of MMAE (with a bridge structure containing two methyl substituents)-alt-MCPBD/MCHBD decreases with the increase in the size of the substituents. This indicates that the structure of DPE and its derivatives affects the aggregation structure of these alternating polymers. Interestingly, DPE and its derivatives (MTAE and MMAE) do not have chiral carbon atoms or stereoisomers in their polymer chains. The amorphous-to-crystalline transition of the polymer was achieved by changing the substituent structure of the dienes and DPE derivatives used in alternating copolymerization. Therefore, the crystallization of these crystalline polymers in CCPIC can be attributed to the steric hindrance effect brought about by the comonomer structure rather than just the stereoregularity of the monomer units in the chain.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


