烯烃环氧化反应和析氧反应在金阳极上竞争活性表面氧原子
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
有机分子在反应电极上部分氧化的速率和选择性取决于反应物质和旁观物质的特性和普遍性。本文研究了在水溶液乙腈(CH3CN)电解液中,水(H2O)对金(Au)的电化学氧化生成活性氧对1-己烯(C6H12)的环氧化反应机理。循环伏安法测量表明,氧(O2)的演化与C6H12的环氧化反应相竞争,在任何一个反应发生之前,Au表面都必须氧化。原位拉曼光谱揭示了活性阳极上的活性氧和旁观者(CH3CN)以及电化学双层内的物质。环氧化的法拉第效率和环氧化物生成速率与O2析出速率之比随C6H12浓度线性增加,与H2O浓度成反比,这与描述C6H12与化学吸附氧原子(O*)之间反应速率的解析表达式一致,排除了其他形式的活性氧(如O2*、OOH*、OH*)的可能性。这些发现表明,环氧化反应和O2演化反应具有一系列共同的步骤,即通过电化学水活化形成O*,然后发散。随后,O*与C6H12通过非法拉第反应生成环氧化物,而O*与H2O通过法拉第反应生成O2,然后去质子化。这些差异导致响应电极电位的速率明显变化,因此,不同的塔菲尔斜率。总的来说,这些结果提供了一个涉及O*反应的C6H12环氧化的自一致机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
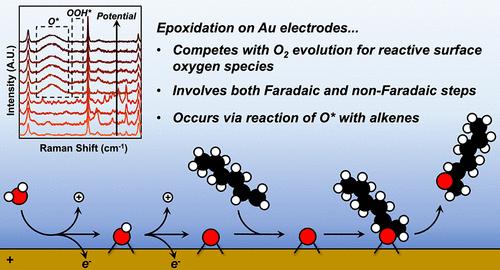
Alkene Epoxidation and Oxygen Evolution Reactions Compete for Reactive Surface Oxygen Atoms on Gold Anodes
Rates and selectivities for the partial oxidation of organic molecules on reactive electrodes depend on the identity and prevalence of reactive and spectator species. Here, we investigate the mechanism for the epoxidation of 1-hexene (C6H12) with reactive oxygen species formed by electrochemical oxidation of water (H2O) on gold (Au) in an aqueous acetonitrile (CH3CN) electrolyte. Cyclic voltammetry measurements demonstrate that oxygen (O2) evolution competes with C6H12 epoxidation, and the Au surface must oxidize before either reaction occurs. In situ Raman spectroscopy reveals reactive oxygen species and spectators (CH3CN) on the active anode as well as species within the electrochemical double layer. The Faradaic efficiencies toward epoxidation and the ratios of epoxide formation to O2 evolution rates increase linearly with the concentration of C6H12 and depend inversely on the concentration of H2O, which agree with analytical expressions that describe rates for reaction between C6H12 and chemisorbed oxygen atoms (O*) and exclude proposals for other forms of reactive oxygen (e.g., O2*, OOH*, OH*). These findings show that the epoxidation and O2 evolution reactions share a set of common steps that form O* through electrochemical H2O activation but then diverge. Subsequently, epoxides form when O* reacts with C6H12 through a non-Faradaic process, whereas O2 evolves when O* reacts with H2O through a Faradaic process to form OOH*, which then deprotonates. These differences lead to distinct changes in rates in response to electrode potential, and hence, disparate Tafel slopes. Collectively, these results provide a self-consistent mechanism for C6H12 epoxidation that involves reactive O*.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


