α,β-不饱和羧酸的立体保持脱羧酰胺化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
胺类化合物已成为胺类化合物的有力替代品,表现出广泛的反应性,可用于进一步的合成修饰,包括亲核加成、环加成和不对称氢化。虽然过渡金属催化的烯基(伪)卤化物与酰胺的交叉偶联已被广泛用于构建这种有价值的支架,但它受到一些限制,例如需要过渡金属催化剂和烯基(伪)卤化物的制备合成。在这项研究中,我们报道了一种温和、方便的立体保持脱羧酰胺化α,β-不饱和羧酸与易于获得的1,4,2-二恶唑-5-酮,提供了一个实用的合成途径。密度泛函理论(DFT)计算揭示了一种合理的反应机制,该反应涉及在二恶唑酮上亲核羧酸盐的加成,然后是顺序的协调重排。本文章由计算机程序翻译,如有差异,请以英文原文为准。
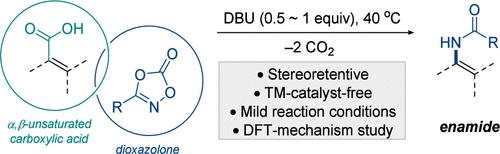
Stereoretentive Decarboxylative Amidation of α,β-Unsaturated Carboxylic Acids to Access Enamides
Enamides have emerged as robust alternatives for enamines, exhibiting versatile reactivity for further synthetic modifications, including nucleophilic addition, cycloaddition, and asymmetric hydrogenation. While transition-metal-catalyzed cross-coupling of alkenyl (pseudo)halides with amides has been widely employed to construct this valuable scaffold, it suffers from some limitations, such as the need for transition-metal catalysts and the preparative synthesis of alkenyl (pseudo)halides. In this study, we report a mild and convenient stereoretentive decarboxylative amidation of α,β-unsaturated carboxylic acids with easily procurable 1,4,2-dioxazol-5-ones, providing a practical synthetic route to enamides. Density functional theory (DFT) calculations revealed a plausible reaction mechanism, which involves the nucleophilic addition of a carboxylate onto dioxazolone, followed by sequential concerted rearrangements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


