路易斯酸介导的可持续质子交换膜电解水的界面水供应
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
催化剂-电解质界面在质子交换膜电解过程中起着至关重要的作用。然而,优化界面氢键以提高催化活性和稳定性仍然是一个重大挑战。本文提出了一种基于硬-软酸碱原理的新型催化剂设计策略,利用硬路易斯酸(LAs = ZrO2, TiO2, HfO2)介导界面氢键重构,从而提高RuO2的酸性出氧反应(OER)性能。机理分析表明,LAs促进了从刚性氢键网络向自由水的定向演化,增强了界面水在RuO2表面的捕获,从而不断向催化位点提供反应物。此外,相互连接的氢键网络促进了质子的快速转移,减少了催化剂表面的局部酸度,防止了结构腐蚀,从而显著提高了长期稳定性。供水和去质子反应的串联途径改变了传统钌基催化剂的溶解机理,强调了其广泛的适用性。因此,ZrO2-RuO2表现出显著降低的过电位170 mV,并具有较高的耐久性,在酸性OER下在10 mA cm-2下维持1800小时,在PEMWE中在2 a cm-2下保持100小时的强劲活性,优于大多数Ru/ ir基催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
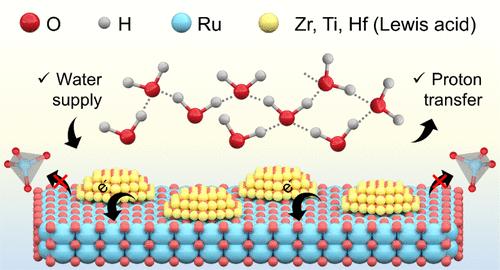
Lewis Acid-Mediated Interfacial Water Supply for Sustainable Proton Exchange Membrane Water Electrolysis
The catalyst–electrolyte interface plays a crucial role in proton exchange membrane water electrolysis (PEMWE). However, optimizing the interfacial hydrogen bonding to enhance both catalytic activity and stability remains a significant challenge. Here, a novel catalyst design strategy is proposed based on the hard–soft acid–base principle, employing hard Lewis acids (LAs = ZrO2, TiO2, HfO2) to mediate the reconfiguration of interfacial hydrogen bonding, thereby enhancing the acidic oxygen evolution reaction (OER) performance of RuO2. Mechanistic analysis indicates that LAs prompt a directional evolution from a rigid hydrogen bonding network to free water, enhancing the trapping of interfacial water on the RuO2 surface, which continuously supplies reactants to the catalytic sites. Moreover, the interconnected hydrogen bonding network facilitates rapid proton transfer, reducing local acidity on the catalyst surface and preventing structural corrosion, thus significantly improving long-term stability. The tandem pathway of water supply and deprotonation transforms the dissolution mechanism of traditional Ru-based catalysts, emphasizing the widespread applicability. Consequently, ZrO2–RuO2 displays a significantly reduced overpotential of 170 mV and exhibits high durability, sustaining 1800 h at 10 mA cm–2 under acidic OER, and maintains robust activity for 100 h at 2 A cm–2 in PEMWE, outperforming most Ru/Ir-based catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


