活细胞中酶活性的空间定量成像
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
酶活性在细胞异质性中起关键作用。它在活细胞中的空间定量成像不仅可以直接显示细胞的异质性,而且可以帮助人们了解细胞的异质性。由于成像探针的细胞内停留时间短或缺乏内部参考,目前的方法很难实现。为此,我们合理设计了一种自参拉曼探针Val-Cit-Cys(StBu)- pra - gy - cbt (ne- cbt),该探针通过细胞内组织蛋白酶B (CTSB)启动的CBT-Cys点击反应,在细胞内产生长时间保留的环状二聚体。同时,利用其两个化学键(C≡C和C≡N)在反应后的拉曼信号变化,对CTSB活性进行自参考和定量拉曼成像。体外实验表明,采用壳隔离纳米粒子增强拉曼光谱技术,20 μM ne- cbt能够定量检测CTSB活性,检出限为61.4 U L-1。在自制的微流控通道下,成功地将ne- cbt应用于活细胞CTSB活性的空间定量成像。我们的策略为人们提供了一种直接和定量显示细胞异质性的简便方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
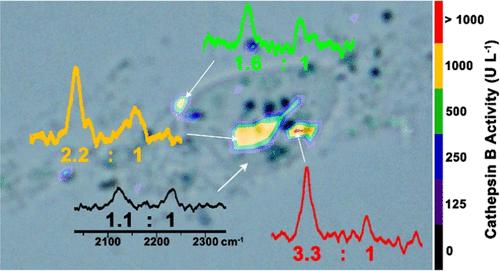
Spatially Quantitative Imaging of Enzyme Activity in a Living Cell
Enzyme activity plays a key role in cell heterogeneity. Its spatially quantitative imaging in a living cell not only directly displays but also helps people to understand cell heterogeneity. Current methods are hard to achieve due to the short intracellular retention or lack of internal reference of the imaging probes. Herein, we rationally designed a self-referenced Raman probe Val-Cit-Cys(StBu)-Pra-Gly-CBT (Yne-CBT) which takes an intracellular cathepsin B (CTSB)-initiated CBT-Cys click reaction to yield a long-retained cyclic dimer in cell. In the meantime, Raman signal changes of its two chemical bonds (C≡C and C≡N) after the reaction are used for self-referencing and quantitative Raman imaging of CTSB activity. In vitro experiments demonstrated that, with shell-isolated nanoparticle-enhanced Raman spectroscopy technique, 20 μM Yne-CBT was able to quantitatively detect CTSB activity with a limit of detection of 61.4 U L–1. Under a homemade microfluidic channel, Yne-CBT was successfully applied for spatially quantitative imaging CTSB activity in a living cell. Our strategy provides people with a facile method to directly and quantitatively display cell heterogeneity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


