9,9′-二色系单晶中有序溶剂分子的极性选择:单重态裂变或对称破缺电荷分离
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
单线态激子裂变(SF)和对称破缺电荷分离(SB-CS)都是发生在两个有机发色团之间的光物理过程,都对提高太阳能转换有重要意义。在这里,我们通过溶剂插层来改变晶体内部的电场,从而调整了SF和SB-CS之间的9,9 ' -二蒽(BA)单晶的光物理性质。BA晶体生长在邻二甲苯、氯苯、邻二氯苯和苯腈中,以及无溶剂的熔体中。通过x射线衍射、稳态光谱学和瞬态吸收显微镜对晶体进行了研究,以阐明插层溶剂分子的作用。结构中无溶剂的晶体发生快速SF (< 2ps),而插入中极性溶剂邻二甲苯、氯苯和邻二氯苯的晶体表现出电荷转移介导的SF。最后,含高极性苯腈的晶体进行了SB-CS而不是SF。这些结果表明,控制BA在晶体结构中的溶剂化可以调节其在SF和SB-CS之间的光物理性质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
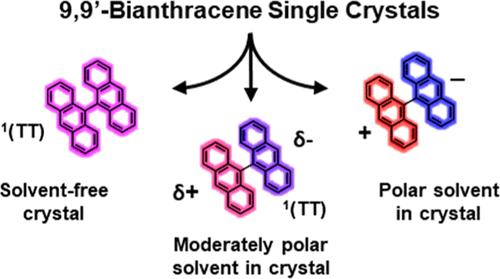
Polarity of Ordered Solvent Molecules in 9,9′-Bianthracene Single Crystals Selects between Singlet Fission or Symmetry-Breaking Charge Separation
Singlet exciton fission (SF) and symmetry-breaking charge separation (SB-CS) are both photophysical processes that can occur between two organic chromophores and are both of interest to improve solar energy conversion. Here, we tuned the photophysics of a 9,9′-bianthracene (BA) single crystal between SF and SB-CS using solvent intercalation to change the electric field within the crystal. Crystals of BA were grown in o-xylene, chlorobenzene, o-dichlorobenzene, and benzonitrile, as well as solvent-free from a melt. The crystals were studied by X-ray diffraction, steady-state optical spectroscopy, and transient absorption microscopy to elucidate the role of the intercalated solvent molecules. The crystals with no solvent in the structure undergo fast SF (<2 ps), while the crystals with intercalated moderately polar solvents o-xylene, chlorobenzene, and o-dichlorobenzene show evidence of charge-transfer-mediated SF. Finally, the crystals containing highly polar benzonitrile undergo SB-CS instead of SF. These results demonstrate that controlling solvation of BA in the crystal structure can tune its photophysics between SF and SB-CS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


