PPAR激动剂bezafbrate对d -2-羟基戊二酸在大鼠脑内诱导的氧化还原和能量稳态破坏、神经元死亡、星形胶质反应性和神经炎症的保护作用
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
d -2-羟基戊二酸尿症的生化标志是d -2-羟基戊二酸(D2HG)的脑蓄积。患者主要表现为神经系统症状,其发病机制尚不清楚。因此,我们研究了幼年大鼠脑内注射这种代谢物引起的D2HG脑水平升高对大脑皮层氧化还原和线粒体稳态以及组织化学标志的影响。D2HG通过增加活性氧和脂质过氧化水平以及超氧化物歧化酶、谷胱甘肽过氧化物酶和谷胱甘肽还原酶的活性和降低还原性谷胱甘肽水平来破坏氧化还原稳态。此外,复合物IV和线粒体肌酸激酶活性以及电压依赖性阴离子通道1、线粒体外膜转座酶20和过氧化物酶体增殖体激活受体-γ共激活因子1-α的蛋白质含量均被D2HG降低,表明生物能量功能障碍和线粒体生物发生中断。D2HG还降低了神经元核蛋白含量,增加了裂解caspase-3、S100钙结合蛋白B、胶质原纤维酸性蛋白和离子钙结合接头分子1,分别表明神经元丢失、凋亡、星形胶质细胞形成和小胶质细胞活化。肿瘤坏死因子α的表达也显著增强,反映炎症反应增加。我们还评估了贝扎贝特(BEZ)预处理是否可以预防D2HG引起的改变。BEZ使大多数d2hg诱导的有害效应正常化。因此,D2HG引起的生物能量学和氧化还原状态破坏,与神经元死亡、胶质反应性和炎症反应增加有关,可能代表了D-2-HGA脑损伤的潜在病理机制。最后,提出BEZ可能潜在地用于治疗D-2-HGA。本文章由计算机程序翻译,如有差异,请以英文原文为准。
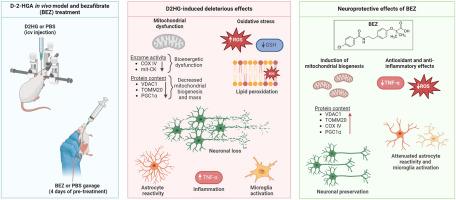
Protective effects of the PPAR agonist bezafibrate against disruption of redox and energy homeostasis, neuronal death, astroglial reactivity, and neuroinflammation induced in vivo by D-2-hydroxyglutaric acid in rat brain
The biochemical hallmark of D-2-hydroxyglutaric aciduria is brain accumulation of D-2-hydroxyglutaric acid (D2HG). Patients present predominantly neurological manifestations, whose pathogenesis is still unknown. Thus, we examined the impact of elevated brain levels of D2HG, induced by intracerebral injection of this metabolite in juvenile rats, on redox and mitochondrial homeostasis and histochemical landmarks in the cerebral cortex. D2HG administration disrupted redox homeostasis by increasing the levels of reactive oxygen species and lipid peroxidation and the activities of superoxide dismutase, glutathione peroxidase, and glutathione reductase and decreasing reduced glutathione levels. Furthermore, the complex IV and mitochondrial creatine kinase activities, as well as the protein contents of voltage-dependent anion channel 1, translocase of outer mitochondrial membrane 20, and peroxisome proliferator-activated receptor-γ coactivator 1-α, were diminished by D2HG, indicating bioenergetics dysfunction and disrupted mitochondrial biogenesis. D2HG also reduced neuronal nuclear protein content and augmented cleaved caspase-3, S100 calcium-binding protein B, glial fibrillary acidic protein, and ionized calcium-binding adaptor molecule 1, indicating neuronal loss, apoptosis, astrogliosis, and microglial activation, respectively. The tumor necrosis factor alpha expression was also significantly augmented, reflecting an increased inflammatory response. We also evaluated whether bezafibrate (BEZ) pretreatment could prevent the alterations induced by D2HG. BEZ normalized most of the D2HG-induced deleterious effects. Therefore, bioenergetics and redox status disruption caused by D2HG, associated with neuronal death, glial reactivity, and increased inflammatory response, may potentially represent pathomechanisms of brain damage in D-2-HGA. Finally, it is proposed that BEZ may be potentially used as therapy for D-2-HGA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


