基团14双根碱可逆二氧化碳捕获和(脱)氧的结构快照
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
虽然与闭壳物质相比,双自由基在激活惰性分子方面应该表现出相当小的反应屏障,但稳定主基双自由基对二氧化碳的激活和功能化实际上尚未被探索。在这项工作中,我们基于阴离子二苯(ADC)框架(ADC = PhC{N(Dipp)C}2;Dipp = 2,6- ipr2c6h3)。[(ADC)E]2很容易与CO2进行[4 + 2]-环加成,从而得到烯型双金属烯[(ADC)E]2(OC = O)。当E = Ge时,CO2的加入是可逆的;因此,CO2在真空或高温下分离并再生[(ADC)Ge]2。[(ADC)Sn]2(OC = O)是可分离的,但是将额外的CO2脱氧形成[(ADC)Sn]2(O2CO)和CO. [(ADC)Si]2(OC = O)是极活泼的,不能被分离或检测到,因为它自发地与CO2进一步反应,产生难以捕捉的单体Si(IV)氧化物[(ADC)Si(O)]2(COn)或碳酸盐[(ADC)Si(CO3)]2(COn) (n = 1或2)通过CO2的(脱)氧化。所有分离化合物的分子结构都已通过x射线衍射确定,并通过DFT计算提出了它们形成的机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
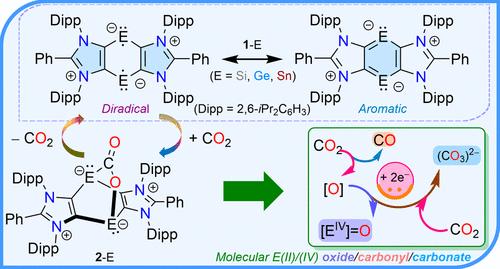
Structural Snapshots of Reversible Carbon Dioxide Capture and (De)oxygenation at Group 14 Diradicaloids
Although diradicals should exhibit a rather small reaction barrier as compared to closed-shell species for activating kinetically inert molecules, the activation and functionalization of carbon dioxide with stable main-group diradicals remain virtually unexplored. In this work, we present a thorough study on CO2 activation, reversible capture, and (de)oxygenation mediated by stable Group 14 singlet diradicals (i.e., diradicaloids) [(ADC)E]2 (E = Si, Ge, Sn) based on an anionic dicarbene (ADC) framework (ADC = PhC{N(Dipp)C}2; Dipp = 2,6-iPr2C6H3). [(ADC)E]2 readily undergo [4 + 2]-cycloadditions with CO2 to result in barrelene-type bis-metallylenes [(ADC)E]2(OC═O). The CO2 addition is reversible for E = Ge; thus, CO2 detaches under vacuum or at an elevated temperature and regenerates [(ADC)Ge]2. [(ADC)Sn]2(OC═O) is isolable but deoxygenates additional CO2 to form [(ADC)Sn]2(O2CO) and CO. [(ADC)Si]2(OC═O) is extremely reactive and could not be isolated or detected as it spontaneously reacts further with CO2 to yield elusive monomeric Si(IV) oxides [(ADC)Si(O)]2(COn) or carbonates [(ADC)Si(CO3)]2(COn) (n = 1 or 2) via the (de)oxygenation of CO2. The molecular structures of all isolated compounds have been established by X-ray diffraction, and a mechanistic insight of their formation has been suggested by DFT calculations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


