硝酸还原高效电合成氨的Cu/Fe3O4@CN串联催化剂。
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电化学硝酸还原反应(ENO3RR)是一种绿色增值制氨工艺,同时在常温条件下处理废水。然而,由于复杂的多重质子和电子转移过程,构建高效的催化剂仍然是一个巨大的挑战。在此,我们报道了一种由n掺杂碳层包覆Cu/Fe3O4异质结构组成的串联催化剂Cu/Fe3O4@CN,用于在碱性介质中高效电催化硝酸还原制氨。在-0.9 V vs. RHE、0.1 M KOH的最佳电位下,该催化剂的氨法拉第效率和氨收率分别高达96.57%和22104.15 μg h-1 mgcat-1。优异的en3rr性能优于大多数已报道的过渡金属基en3rr电催化剂。更重要的是,Cu/Fe3O4@CN在200 mA cm-2的大电流密度下也表现出优异的稳定性,并且在连续电解36 h时具有较长的耐久性。通过Cu/Fe3O4@CN中Cu和Fe3O4两组分的协同作用,验证了NO3-→NO2-→NH3的串联催化机理。其中Cu具有较强的NO3-吸附能力,可以还原为NO2-,而Fe3O4可以促进水解离生成丰富的活性*H,保证加氢过程的顺利进行,同时抑制竞争性析氢反应(HER)的发生。Cu和Fe3O4之间异质结构的形成也显著降低了速率决定步骤(*NO→*NOH)的能垒,从而使Cu/Fe3O4@CN具有较高的性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
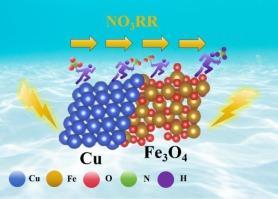
A Cu/Fe3O4@CN tandem catalyst for efficient ammonia electrosynthesis from nitrate reduction
Electrochemical nitrate reduction reaction (ENO3RR) is a green technology for value-added ammonia production meanwhile treating waste water at ambient conditions. However, it remains a great challenge to construct efficient catalysts due to the complex multiple proton and electron transfer process. Herein, we report a tandem catalyst Cu/Fe3O4@CN composed of N-doped carbon layer coated Cu/Fe3O4 heterostructure toward highly efficient electrocatalytic nitrate reduction to ammonia production in alkaline medium. The catalyst can achieve an ammonia Faradaic efficiency and ammonia yield rate as high as 96.57 % and 22104.15 μg h−1 mgcat−1 at the optimal potential of −0.9 V vs. RHE in 0.1 M KOH. The outstanding ENO3RR performance outperforms the most of reported transitional metal-based ENO3RR electrocatalysts. More importantly, Cu/Fe3O4@CN also exhibits excellent stability even under large current density of 200 mA cm−2, and long-time durability for continuous electrolysis for 36 h. The experimental and theoretical calculations verify the tandem catalysis mechanism of NO3− → NO2− → NH3 through the synergism of the two components Cu and Fe3O4 in Cu/Fe3O4@CN. Specifically, Cu has strong NO3− adsorption capacity which can be reduced into NO2−, while Fe3O4 can boost water dissociation to generate abundant active *H, ensuring the smooth hydrogenation process while suppressing the occurrence of competitive hydrogen evolution reactions (HER). The heterostructure formation between Cu and Fe3O4 also significantly reduces the energy barrier of the rate-determining step (*NO → *NOH), which results in the high performance of Cu/Fe3O4@CN.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


