氟团簇富勒烯:通过单氟原子掺杂调整金属-金属键和磁性能
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
内嵌富勒烯以其特殊的承载能力而闻名,这些能力包含独特的键基序。在这项研究中,我们报告了一个简单的策略来合成一个新的簇富勒烯家族,氟簇富勒烯(FCFs)。这项工作表明,锕系元素和稀土金属以及碱土金属可以被封装在各种富勒烯笼中,并且这些富勒烯可以在没有额外功能化方法的情况下以原始形式获得。值得注意的是,Th2F@Ih(7)-C80和CaScF@Cs(6)-C82被分离出来,并通过x射线单晶衍射和多光谱技术以及DFT计算对它们的分子结构和磁性进行了表征。这些发现表明,单个氟原子的独特内部添加显着改变了Th-Th和Ca-Sc的金属-金属键相互作用。虽然Th2@Ih(7)-C80具有σ2 Th-Th键,但在Th2F@Ih(7)-C80内部加入氟化物后,形成了前所未有的锕系-锕系(Th-Th)单电子金属-金属键。同样,虽然在CaSc@Cs(6)-C82中存在Ca-Sc单键,但其表现出优异的分子量子比特特性,氟的加入使该化合物转变为单线态。本研究不仅成功合成了一个新的fcf家族,这可能是一个广泛的家族,它还表明氟掺杂可以诱导新的金属-金属键基序,从而导致潜在的有趣的磁性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
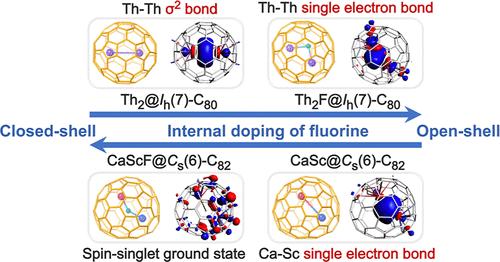
Fluoride Clusterfullerenes: Tuning Metal–Metal Bonding and Magnetic Properties via Single Fluorine Atom Doping
Endohedral fullerenes are known for their exceptional ability to host metal clusters that contain unique bonding motifs. In this study, we report a facile strategy to synthesize a new family of clusterfullerenes, fluoride clusterfullerenes (FCFs). This work demonstrates that actinides and rare earth metals as well as alkaline earth metals can be encapsulated within a variety of fullerene cages, and these fullerenes can be obtained in their pristine form without additional functionalization methods. Notably, Th2F@Ih(7)-C80 and CaScF@Cs(6)-C82 were isolated and their molecular structures and magnetic properties were characterized by X-ray single-crystal diffraction and multiple spectroscopic techniques as well as DFT calculations. These findings reveal that the unique internal addition of a single fluorine atom significantly alters the metal–metal bonding interactions of Th–Th and Ca–Sc. While Th2@Ih(7)-C80 hosts a σ2 Th–Th bond, an unprecedented actinide–actinide (Th–Th) single electron metal–metal bond is formed inside Th2F@Ih(7)-C80 upon the internal addition of fluoride. Similarly, while a Ca–Sc single electron bond exists in CaSc@Cs(6)-C82, which exhibits excellent molecular qubit properties, the addition of fluoride transforms the compound into a singlet. The present study not only highlights the successful synthesis of a novel family of FCFs, which will likely be an extensive family, it also shows that fluorine doping can induce novel metal–metal bonding motifs leading to potentially intriguing magnetic properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


