靶向纳米颗粒的溶酶体-线粒体级联驱动癌症免疫治疗的强猝灭
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
货物的亚细胞分布在决定细胞命运和治疗效果方面起着至关重要的作用。然而,实现治疗药物的精确递送到特定的细胞内靶点仍然是一个重大的挑战。在这里,我们提出了一个三模酸/酶控纳米平台(TAEN),它在酸性核内体中被分解,然后被溶酶体组织蛋白酶B切割,以促进释放的货物有效和有针对性地运输到线粒体室。通过利用这种纳米载体,我们成功地将光敏剂分子选择性地分选到线粒体中,共定位系数高达0.98,导致线粒体内特异性产生活性氧胁迫,用于有效的基于热释作用的癌症治疗。线粒体应激的诱导触发了固有的凋亡途径以及caspase-3/ gasderin - e (GSDME)级联,导致癌细胞杀伤效果比溶酶体应激提高了近2个数量级。此外,由于其刺激先天和适应性免疫反应的卓越能力,我们的线粒体分选纳米光敏剂在多种荷瘤小鼠模型中显示出强大的抗肿瘤免疫功效。该研究不仅为亚细胞靶向递送的工程纳米药物提供了见解,而且为亚细胞分辨率纳米生物学领域的高级研究提供了有价值的工具包。本文章由计算机程序翻译,如有差异,请以英文原文为准。
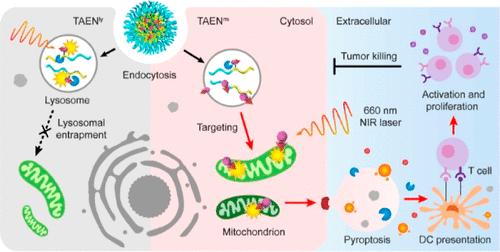
Lysosome-Mitochondria Cascade Targeting Nanoparticle Drives Robust Pyroptosis for Cancer Immunotherapy
The subcellular distribution of cargoes plays a crucial role in determining cell fate and therapeutic efficacy. However, achieving the precise delivery of therapeutics to specific intracellular targets remains a significant challenge. Here, we present a trimodular and acid/enzyme-gated nanoplatform (TAEN) that undergoes disassembly within acidic endosomes and then is cleaved by lysosomal cathepsin B to facilitate efficient and targeted transport of released cargoes into mitochondria compartments. By utilizing this nanovehicle, we successfully achieve selective sorting of photosensitizer molecules into mitochondria with a colocalization coefficient of up to 0.98, leading to the generation of reactive oxygen species stress specifically within the mitochondria for potent pyroptosis-based cancer therapy. The induction of mitochondrial stress triggers the intrinsic apoptotic pathway as well as caspase-3/gasdermin-E (GSDME) cascade, resulting in an enhanced cancer cell killing efficacy by nearly 2 orders of magnitude as compared to lysosomal stress. Furthermore, due to its superior capability to stimulate both innate and adaptive immune responses, our mitochondria-sorted nanophotosensitizer exhibits robust antitumor immune efficacy in multiple tumor-bearing mice models. This study not only provides insights into engineering nanomedicines for subcellular targeted delivery but also offers a valuable toolkit for advanced research in the field of nanobiology at subcellular resolution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


